Identification of Absorbed Constituents in the Rabbit Plasma and Cerebrospinal Fluid after Intranasal Administration of Asari Radix et Rhizoma by HS-SPME-GC-MS and HPLC-APCI-IT-TOF-MSn
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Absorbed Constituents in Rabbit Plasma and CSF by HS-SPME-GC-MS
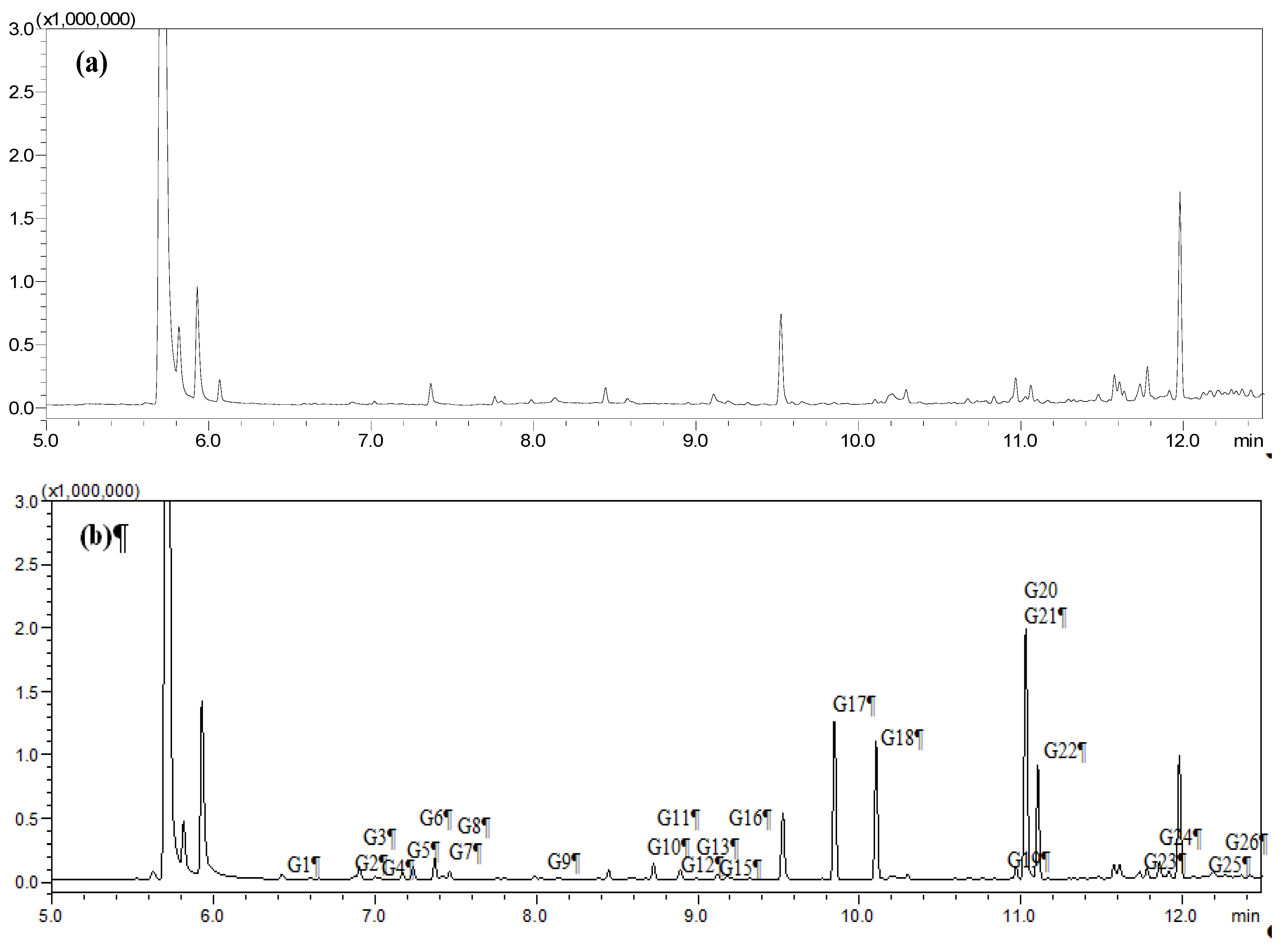



2.2. Identification of Absorbed Constituents in Rabbit Plasma and CSF by HPLC-APCI-IT-TOF-MSn
| Compound No. | tR (min) | RI b [12] | Constituents | Group b | Group c | ||
|---|---|---|---|---|---|---|---|
| Plasma | CSF | Plasma | CSF | ||||
| G1 | 6.423 | 944 | α-Pinene | + | + | − | − |
| G2 | 6.853 | 982 | Sabinene | + | + | − | − |
| G3 | 6.902 | 988 | β-Pinene | + | + | − | − |
| G4 | 7.000 | 993 | Myrcene | + | + | − | − |
| G5 | 7.170 | 1012 | α-Phellandrene | + | + | − | − |
| G6 | 7.236 | 1018 | 3-Carene | + | + | − | − |
| G7 | 7.421 | 1035 | Limonene | + | + | − | − |
| G8 | 7.463 | 1040 | Eucalyptol | + | + | − | − |
| G9 | 8.031 | 1096 | Terpinolene | + | + | − | − |
| G10 | 8.675 | 1158 | Camphor | + | + | − | − |
| G11 a | 8.724 | 1162 | Eucarvone | + | + | + | + |
| G12 a | 8.890 | 1178 | l-Borneol | + | + | + | + |
| G13 | 8.992 | 1188 | Terpinen-4-ol | + | + | − | − |
| G14 | 9.035 | 1192 | p-Cymen-8-ol | − | + | − | − |
| G15 | 9.118 | 1200 | α-Terpineol | + | + | − | − |
| G16 | 9.179 | 1206 | Estragole | + | − | − | − |
| G17 a | 9.847 | 1275 | 3,5-Dimethoxytoluene | + | + | + | + |
| G18 a | 10.105 | 1300 | Safrole | + | + | + | − |
| G19 | 10.971 | 1400 | Tetradecane | + | + | − | − |
| G20 a, G21 a | 11.030 | 1408 | 3,4,5-Trimethoxy-toluene/methyleugenol | + | + | + | + |
| G22 a | 11.106 | 1417 | 2,3,5-Trimethoxytoluene | + | + | + | + |
| G23 | 11.783 | 1501 | Pentadecane | + | + | − | − |
| G24 | 11.860 | 1510 | Asaricin | + | + | + | − |
| G25 | 12.191 | 1550 | 3,4-Methylenedioxy-propiophenone | + | + | − | − |
| G26 | 12.268 | 1560 | Elemicin | + | + | − | − |
| Sum | 25 | 25 | 8 | 6 | |||

| No. | tR (min) | Meas. (Da) | Pred. (Da) | Err. | DBE | Formula | Identification results | Characteristic fragment ions | Group b | Group c | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (ppm) | P | C | P | C | ||||||||
| L1 | 11.486 | 359.1501 | 359.1489 | 3.34 | 10 | C20H22O6 | Epipinoresinol isomer | 341.1380, 323.1279, 291.1026, 271.1010, 259.0748, 137.0618 | + | − | − | − |
| L2 | 14.864 | 359.1489 | 359.1489 | 0 | 10 | C20H22O6 | Epipinoresinol isomer | 341.1373, 323.1263, 291.1021, 271.0964, 259.0781, 137.0620 | + | − | − | − |
| L3 | 22.813 | 357.1325 | 357.1333 | −2.24 | 11 | C20H20O6 | Xanthoxylol isomer | 339.1218, 321.1155, 291.0999, 289.0903, 269.0786, 137.0464, 135.0417 | + | − | − | − |
| L4 | 26.497 | 211.0955 | 211.0965 | −4.74 | 5 | C11H14O4 | 3,4-Dimethoxybenzenepropionic acid | 193.0846, 178.0611, 165.0904, 161.0598, 133.0630 | + | − | − | − |
| L5 a | 27.031 | 359.1489 | 359.1489 | 0 | 10 | C20H22O6 | Epipinoresinol | 341.1372, 323.1263, 291.1021, 271.0946, 259.0781, 137.0620 | + | − | − | − |
| L6 a | 31.577 | 183.1007 | 183.1016 | −4.92 | 4 | C10H14O3 | 3,4,5-Trimethoxytoluene | 168.0772, 152.0825, 151.0765 | + | + | − | − |
| L7 a | 35.188 | 183.1007 | 183.1016 | −4.92 | 4 | C10H14O3 | 2,3,5-Trimethoxytoluene | 168.0772, 152.0825, 151.0723 | + | + | − | − |
| L8 | 36.811 | 387.1428 | 387.1438 | −2.58 | 11 | C21H22O7 | (1R,2S,5R,6R)-5´-O-Methylpluviatilol | 369.1354, 351.1237, 339.1198, 319.0954, 299.0947, 167.0723, 135.0462 | + | − | − | − |
| L9 a | 37.013 | 209.08 | 209.0808 | −3.83 | 6 | C11H12O4 | Kakuol methyl ether | 191.0710, 176.0459, 161.0614, 133.0621 | + | − | − | − |
| L10 a | 38.435 | 357.1333 | 357.1333 | 0 | 11 | C20H20O6 | Xanthoxylol | 339.1222, 321.1147, 291.1048, 289.0874, 269.0807, 137.0545, 135.0423 | + | − | − | − |
| L11 a | 38.733 | 195.0644 | 195.0652 | −4.1 | 6 | C10H10O4 | Kakuol | 177.0522, 147.0418, 139.0386, 137.0179, 119.0478, 109.0261 | + | − | − | − |
| L12 | 39.104 | 197.0804 | 197.0808 | −2.03 | 5 | C10H12O4 | Hydroferulic acid | 180.0751, 179.0720, 155.0732, 151.0756, 133.0620, 123.0894 | + | − | − | − |
| L13 | 51.123 | 222.1842 | 222.1852 | −4.5 | 4 | C14H23NO | Spilanthol | 167.1237, 166.1329, 152.0970, 149.0985, 123.1139, 121.1017, 81.0807 | + | − | − | − |
| L14 | 51.317 | 355.1191 | 355.1176 | 4.22 | 12 | C20H18O6 | l-Sesamin | 337.1068, 319.0966, 289.0837, 261.0917, 231.0791, 203.0856, 135.0418 | + | − | − | − |
| L15 a | 54.163 | 355.1178 | 355.1176 | 0.56 | 12 | C20H18O6 | l-asarinin | 337.1075, 319.0978, 289.0875, 261.0916, 231.0784, 203.0850, 135.0425 | + | + | − | − |
| L16 | 56.775 | 248.2016 | 248.2009 | 2.82 | 5 | C16H25NO | N-isobutyl-2E,4E,8Z,10Z-dodecatetraenamide | 192.1362, 175.1124, 167.1304, 166.1217, 149.1325, 147.1173, 142.1218, 121.1007, 107.0847 | + | + | + | − |
| L17 | 57.218 | 248.2005 | 248.2009 | −1.61 | 5 | C16H25NO | N-isobutyl-2E,4E,8Z,10E-dodecatetraenamide | 192.1362, 175.1124, 167.1289, 166.1232, 149.1325, 147.1174, 142.1206, 121.0999, 107.0852 | + | + | + | − |
| L18 | 58.08 | 248.1997 | 248.2009 | −4.83 | 5 | C16H25NO | N-isobutyl-2,4,8,10-dodecatetraenamide isomer | 192.1452, 175.1116, 167.1284, 166.1255, 149.1296, 147.1160, 142.1191, 121.1002, 107.0820 | + | − | − | − |
| L19 | 61.053 | 248.2004 | 248.2009 | −2.01 | 5 | C16H25NO | N-isobutyl-2,4,8,10-dodecatetraenamide isomer | 175.1135, 167.1339, 149.1333, 147.1138, 133.0672, 121.1017, 107.0813 | + | − | − | − |
| L20 | 62.647 | 250.215 | 250.2165 | −5.99 | 4 | C16H27NO | N-isobutyl-2,4,8-dodecatrienamide | 194.1625, 177.1164, 167.1302, 152.1070, 149.1398, 109.0863, 95.0789 | + | − | − | − |
| L21 | 63.995 | 274.2164 | 274.2165 | −0.36 | 6 | C18H27NO | N-isobutyl-2,4,8,10,12-tetradecapentaenamide | 201.1332, 175.1411, 173.1332 | + | − | − | − |
| L22 | 66.168 | 276.2322 | 276.2322 | 0 | 5 | C18H29NO | N-isobutyl-2,4,8,10-tetradecatetraenamide | 220.1623, 203.1457, 177.1611, 175.1431, 167.1297, 135.1181, 133.0970 | + | − | − | − |
| L23 | 66.563 | 252.2317 | 252.2322 | −1.98 | 3 | C16H29NO | N-isobutyl-2,4-dodecadienamide | 196.1744, 179.1256, 154.1227, 95.0480 | + | − | − | − |
| Sum | 23 | 5 | 2 | 0 | ||||||||

2.2.1. Fragmentation Behaviors of Four Reference Compounds in APCI-MSn
2.2.2. Characterization of Eight Absorbed Lignans of AR by HPLC-APCI-IT-TOF-MSn
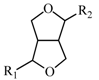 | ||||
|---|---|---|---|---|
| R1 | R2 | R1 diagnostic ion | R2 diagnostic ion | MS base peak ion [M+H−H2O]+ |
 |  | 135.04 (C8H7O2) | 135.04 (C8H7O2) | 337.10 (C20H17O5) |
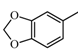 | 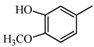 | 135.04 (C8H7O2) | 137.06 (C8H9O2) | 339.12 (C20H19O5) |
 |  | 137.06 (C8H9O2) | 137.06 (C8H9O2) | 341.14 (C20H21O5) |
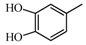 | 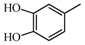 | 123.04 (C7H7O2) | 123.04 (C7H7O2) | 313.10 (C18H17O5) |
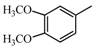 | 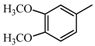 | 151.07 (C9H11O2) | 151.07 (C9H11O2) | 369.17 (C22H25O5) |
 | 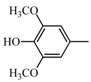 | 137.06 (C8H9O2) | 167.07 (C9H11O3) | 371.15 (C21H23O6) |
 |  | 135.04 (C8H7O2) | 167.07 (C9H11O3) | 369.13 (C21H21O6) |
 | 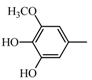 | 135.04 (C8H7O2) | 153.05 (C8H9O3) | 355.12 (C20H19O6) |
2.2.3. Characterization of Nine Absorbed N-alkylamides of AR by HPLC-APCI-IT-TOF-MSn
2.2.4. Characterization of Four Absorbed Phenylpropanoids of AR by HPLC-APCI-IT-TOF-MSn


2.2.5. Characterization of Two Absorbed Benzene Derivatives of AR by HPLC-APCI-IT-TOF-MSn
2.3. Bioactivities of the Absorbed Constituents Related to the Pharmacological Effects of AR
| GC-MS | LC-MS | Same Constituents Identified by GC-MS and LC-MS | Total No. | |
|---|---|---|---|---|
| Plasma | 25 | 23 | 2 (L6 = G20, L7 = G22) | 46 |
| CSF | 25 | 5 | 2 (L6 = G20, L7 = G22) | 28 |
| Same constituents in plasma and CSF | 24 (G1–G13, G15, G17–G26) | 5 (L6, L7, L15–L17) | 2 (L6 = G20, L7 = G22) | 27 |
| Total No. | 26 | 23 | 2 | All: 47 |
| Activity (number) | Structure Type (Number) | Constituents |
|---|---|---|
| Analgesic (14) | Monoterpene (9) | α-Pinene, β-Pinene, Myrcene, α-Phellandrene, Limonene, Eucalyptol, Camphor, l-Borneol, α-Terpineol |
| Phenylpropanoid (3) | Estragole, methyleugenol, 3,4-Dimethoxybenzenepropionic acid | |
| N-alkylamide (2) | Spilanthol, N-Isobutyl-2E,4E,8Z,10E-dodecatetraenamide | |
| Anti-inflammatory (27) | Monoterpene (12) | α-Pinene, Sabinene, β-Pinene, Myrcene, 3-Carene, Limonene, Eucalyptol, Camphor, Eucarvone, l-Borneol, Terpinen-4-ol, α-Terpineol |
| Phenylpropanoid (5) | Estragole, Methyleugenol, Elemicin, Kakuol, Hydroferulic Acid | |
| Benzene derivative (1) | 3,4,5-Trimethoxytoluene | |
| Lignan (4) | Epipinoresinol, (1R,2S,5R,6R)-5´-O-Methylpluviatilol, l-Sesamin, l-Asarinin | |
| N-Alkylamide (5) | Spilanthol, N-Isobutyl-2E,4E,8Z,10Z-dodecatetraenamide, N-Isobutyl-2E,4E,8Z,10E-dodecatetraenamide, N-Isobutyl-2,4,8-dodecatrienamide, N-Isobutyl-2,4-dodecadienamide | |
| Sedative (5) | Monoterpene (3) | Myrcene, Limonene, Eucalyptol |
| Phenylpropanoid (1) | Methyleugenol | |
| Benzene derivative (1) | 3,5-Dimethoxytoluene | |
| Anti-spasmodic (9) | Monoterpene (7) | Camphor, l-Borneol, β-Pinene, α-Phellandrene, Eucalyptol, Terpinolene, α-Terpineol |
| Phenylpropanoid (2) | Methyleugenol, Estragole | |
| Anti-allergic (8) | Monoterpene (2) | Limonene, l-Borneol |
| Phenylpropanoid (2) | Methyleugenol, Elemicin | |
| Lignan (3) | Xanthoxylol, l-Sesamin, l-Asarinin | |
| N-alkylamide (1) | N-Isobutyl-2E,4E,8Z,10E-dodecatetraenamide | |
| Cardiovascular (14) | Monoterpene (9) | α-Pinene, β-Pinene, Terpinen-4-ol, α-Terpineol, Eucalyptol, Camphor, l-Borneol, Limonene, Terpinolene |
| Phenylpropanoid (5) | Methyleugenol, Elemicin, Kakuol, Hydroferulic Acid, Estragole | |
| Antitussive (8) | Monoterpene (4) | α-Pinene , Camphor, Eucalyptol, Terpinen-4-ol |
| Phenylpropanoid (1) | Kakuol | |
| N-alkylamide (3) | N-Isobutyl-2E,4E,8Z,10Z-dodecatetraenamide, N-Isobutyl-2E,4E,8Z,10E -dodecatetraenamide, N-Isobutyl-2,4,8,10,12-tetradecapentaenamide | |
| Hypothermic (2) | Monoterpene (1) | Limonene |
| Phenylpropanoid (1) | Methyleugenol | |
| Anticonvulsant (5) | Monoterpene (4) | Myrcene, Limonene, Terpinen-4-ol, α-Terpineol |
| Phenylpropanoid (1) | Methyleugenol |
3. Experimental
3.1. Reagents and Materials
3.2. Preparation of AR EtOAc Extract
3.3. Animals and Sample Collection
3.4. Automated HS-SPME-GC-MS Analysis
3.5. HPLC-APCI-IT-TOF-MSn Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, S.Z. Zhongyi Biliaofa Quanshu A Comprehensive Treatise on Nasal Therapy of Traditional Chinese Medicine, 1st ed.; Huaxia Publishing House: Beijing, China, 1994; pp. 33–401. [Google Scholar]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar]
- Yu, L.; Chen, Q.L.; Guo, S.S.; Liu, Y.D. Overview on intranasal delivery of Chinese medicine in curing systemic diseases. Asia Pac. Trad. Med. 2008, 4, 88–91. [Google Scholar]
- Feng, J.; Li, F.; Zhao, Y.; Feng, Y.; Abe, Y. Brain pharmacokinetics of tetramethylpyrazine after intranasal and intravenous administration in awake rats. Int. J. Pharm. 2009, 375, 55–60. [Google Scholar] [CrossRef]
- Yao, Z.; Lu, Y.; Du, S.; Chen, X.; Wang, Y. Study on pharmacokinetics of jasminoidin in rabbits administered Xingnaojing Naristillae by nasal medication. Chin. J. Chin. Mater. Med. 2010, 35, 1871–1873. [Google Scholar]
- Gong, Z.N.; Xu, L.Y.; Song, J.Z.; Tao, J.S.; Ma, S.R. Study on rat nasal administration with emulsion of Chinese herb Angelica Dahurica. Chin. J. Clin. Pharm. 2001, 10, 370–373. [Google Scholar]
- Guo, X.J.; Li, J.Y.; Lin, C.Y.; Yang, F. Exploration on nasal administration of traditional Chinese medicine for treating brain diseases. J. Guangdong Pharm. Coll. 2011, 27, 211–214. [Google Scholar]
- Zhao, B.C.; Wu, H.Y. Study on the prescribing rules of traditional Chinese medicines for the treatment of migraine by intranasal administration. Asia Pac. Trad. Med. 2010, 6, 132–133. [Google Scholar]
- Han, A.R.; Kim, H.J.; Shin, M.; Hong, M.; Kim, Y.S.; Bae, H. Constituents of Asarum sieboldii with inhibitory activity on lipopolysaccharide (LPS)-induced NO production in BV-2 microglial cells. Chem. Biodivers. 2008, 5, 346–351. [Google Scholar] [CrossRef]
- Wagner, H.; Bauer, R.; Melchart, D.; Xiao, P.G.; Staudinger, A. Chromatographic Fingerprint Analysis of Herbal Medicines, 2nd ed.; Springer: Vienna, Austria, 2011; Voleme 1, pp. 45–58. [Google Scholar]
- Yuan, X.Q.; Sun, L.F.; Zheng, J. Analgesic components of Asarum and its mechanisms. Shanghai J. Trad. Chin. Med. 2009, 43, 72–75. [Google Scholar]
- Li, C.; Xu, F.; Cao, C.; Shang, M.Y.; Zhang, C.Y.; Yu, J.; Liu, G.X.; Wang, X.; Cai, S.Q. Comparative analysis of two species of Asari Radix et Rhizoma by electronic nose, headspace GC-MS and chemometrics. J. Pharm. Biomed. Anal. 2013, 85, 231–238. [Google Scholar] [CrossRef]
- Yan, G.; Li, Q.; Tan, H.; Ge, T. Electrospray ionization ion-trap time-of-flight tandem mass spectrometry of two furofurans: sesamin and gmelinol. Rapid Commun. Mass Spectrom. 2007, 21, 3613–3620. [Google Scholar] [CrossRef]
- Struijs, K.; Vincken, J.P.; Gruppen, H. Comparison of atmospheric pressure chemical ionization and electrospray ionization mass spectrometry for the detection of lignans from sesame seeds. Rapid Commun. Mass Spectrom. 2008, 22, 3615–3623. [Google Scholar] [CrossRef]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.A.; Smeds, A.I.; Sjöholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2008, 43, 97–107. [Google Scholar]
- Xing, J.; Zhang, S.Q.; Zhong, D.F.; Jia, J.M. Rapid identification of Acanthopanax senticosus mixture by liquid chromatography-electrospray ion trap mass spectrometry. J. Chin. Mass Spectrom. Soc. 2004, 25, 198–203. [Google Scholar]
- Quang, T.H.; Ngan, N.T.; Minh, C.V.; Kiem, P.V.; Tai, B.H.; Thao, N.P.; Song, S.B.; Kim, Y.H. Anti-inflammatory and PPAR transactivational effects of secondary metabolites from the roots of Asarum sieboldii. Bioorg. Med. Chem. Lett. 2012, 22, 2527–2533. [Google Scholar] [CrossRef]
- Boonen, J.; Baert, B.; Burvenich, C.; Blondeel, P.; de Saeger, S.; de Spiegeleer, B. LC–MS profiling of N-alkylamides in Spilanthes acmella extract and the transmucosal behaviour of its main bio-active spilanthol. J. Pharm. Biomed. Anal. 2010, 53, 243–249. [Google Scholar] [CrossRef]
- Thomsen, M.O.; Fretté, X.C.; Christensen, K.B.; Christensen, L.P.; Grevsen, K. Seasonal variations in the concentrations of lipophilic compounds and phenolic acids in the roots of Echinacea purpurea and Echinacea pallida. J. Agric. Food Chem. 2012, 60, 12131–12141. [Google Scholar]
- Mudge, E.; Lopes-Lutz, D.; Brown, P.; Schieber, A. Analysis of alkylamides in Echinacea plant materials and dietary supplements by ultrafast liquid chromatography with diode array and mass spectrometric detection. J. Agric. Food Chem. 2011, 59, 8086–8094. [Google Scholar] [CrossRef]
- Cech, N.B.; Eleazer, M.S.; Shoffner, L.T.; Crosswhite, M.R.; Davis, A.C.; Mortenson, A.M. High performance liquid chromatography/electrospray ionization mass spectrometry for simultaneous analysis of alkamides and caffeic acid derivatives from Echinacea purpurea extracts. J. Chromatogr. A 2006, 1103, 219–228. [Google Scholar]
- Yasuda, I.; Takeya, K.; Itokawa, H. Structures of Amides from Asiasarum heterotropoides MAEK. var. mand shuricum MAEK. Chem. Pharm. Bull. 1981, 29, 564–566. [Google Scholar] [CrossRef]
- Zhang, S.X.; Tani, T.; Yamaji, S.; Gao, X.L.; Wang, X.; Cai, S.Q.; Zhao, Y.Y. Studies on chemical constituents from radix and rhizome of Asarum longerhizomatosum. Chin. Trad. Herba. Drugs 2002, 33, 297–299. [Google Scholar]
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds G11, G12, G17, G18, G20 (L6), G21, G22 (L7), L5, L9, L10, L11, and L15 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, C.; Xu, F.; Xie, D.-M.; Jing, Y.; Shang, M.-Y.; Liu, G.-X.; Wang, X.; Cai, S.-Q. Identification of Absorbed Constituents in the Rabbit Plasma and Cerebrospinal Fluid after Intranasal Administration of Asari Radix et Rhizoma by HS-SPME-GC-MS and HPLC-APCI-IT-TOF-MSn. Molecules 2014, 19, 4857-4879. https://doi.org/10.3390/molecules19044857
Li C, Xu F, Xie D-M, Jing Y, Shang M-Y, Liu G-X, Wang X, Cai S-Q. Identification of Absorbed Constituents in the Rabbit Plasma and Cerebrospinal Fluid after Intranasal Administration of Asari Radix et Rhizoma by HS-SPME-GC-MS and HPLC-APCI-IT-TOF-MSn. Molecules. 2014; 19(4):4857-4879. https://doi.org/10.3390/molecules19044857
Chicago/Turabian StyleLi, Chen, Feng Xu, De-Mei Xie, Yu Jing, Ming-Ying Shang, Guang-Xue Liu, Xuan Wang, and Shao-Qing Cai. 2014. "Identification of Absorbed Constituents in the Rabbit Plasma and Cerebrospinal Fluid after Intranasal Administration of Asari Radix et Rhizoma by HS-SPME-GC-MS and HPLC-APCI-IT-TOF-MSn" Molecules 19, no. 4: 4857-4879. https://doi.org/10.3390/molecules19044857





