Abstract
Aryl-keto-containing α-amino acids are of great importance in organic chemistry and biochemistry. They are valuable intermediates for the construction of hydroxyl α-amino acids, nonproteinogenic α-amino acids, as well as other biofunctional components. Friedel-Crafts acylation is an effective method to prepare aryl-keto derivatives. In this review, we summarize the preparation of aryl-keto containing α-amino acids by Friedel-Crafts acylation using acidic α-amino acids as acyl-donors and Lewis acids or Brönsted acids as catalysts.
1. Introduction
Aryl-keto functional groups are of great importance in organic and biological chemistry. α-Amino acids containing aryl-keto functional groups have emerged as versatile and important building blocks for the synthesis of biological compounds, pharmaceutical intermediates, and natural products for several decades [1,2,3,4,5] (Scheme 1). By utilizing optically pure α-amino acids, which act as multifunctional and commercially available chiral pools, it is possible to prepare optically pure compounds.
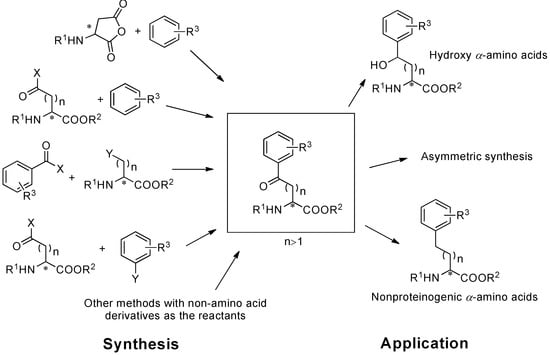
Scheme 1.
Synthesis and application of aryl-keto α-amino acids.
Scheme 1.
Synthesis and application of aryl-keto α-amino acids.
Recently, the synthesis of aryl-keto α-amino acids using methods that are straightforward and convenient has attracted more attention. To this end, numerous methods have been developed, such as nucleophilic substitution [6,7], catalytic alkylations [8,9], and Michael additions [10] and so on. Generally, these methods require the preparation of special reagents and expensive catalysts while rigorous conditions are also necessary. Furthermore, the chiral resolution of the enantiomers creates difficulties in the application of these strategies. Friedel-Crafts acylation [11], a method used to acylate aromatic rings, has been widely used in organic synthesis. The corresponding products are less reactive than the original compounds due to the electron-withdrawing effects of the carbonyl group, which hinder multiple acylations. Due to the resonance effect, no carbocation rearrangements occur during the reaction. Construction of aryl-keto α-amino acids by Friedel-Crafts acylation has been carried out for many years [12,13,14]. Through the reaction of aromatics and a side chain of α-amino acids derivatives, it is easy to synthesize optically pure α-amino acids containing enantiomer skeletons. Hydroxy α-amino acids and non-proteinogenic α-amino acids can be easily prepared, followed by further hydrogenolysis and deprotection. In addition, the enantiomer configuration can be well controlled by using chiral α-amino acids as acyl-donors, which is useful for chiral and asymmetric synthesis. It is well known that catalysts such as Lewis acids and Brönsted acids are essential for Friedel-Crafts acylation. The preparation of aryl-keto α-amino acids is mainly carried out by the catalysis of AlCl3, hydrofluoric acid (HF), or triflic acid (TfOH). This review focuses on the strategies used for the construction of aryl-keto α-amino acids by Friedel-Crafts acylation using different catalysts, aiming to provide a comprehensive discussion about alternative methods for the preparation of aryl-keto α-amino acids as well as their derivatives.
2. General Methods to Prepare Aryl-keto α-Amino Acid Derivatives
In this article, we briefly discuss the general methods used to prepare aryl-keto α-amino acid derivatives. The strategies are categorized based on the use of α-amino acid derivatives in the reactions.
2.1. α-Amino Acid Derivatives as the Reactants
The Claisen condensation is an effective method for the preparation of β-keto esters [15]. Reactions of α-amino acid derivatives such as glycine Schiff base esters (1, Scheme 2A), isocyano esters (2, Scheme 2B), or glycine derivatives (3, Scheme 2C) with benzyl chloride or aryl anhydride analogs in the presence of bases are procedures for the construction for aryl-keto α-amino acid derivatives [16,17,18].
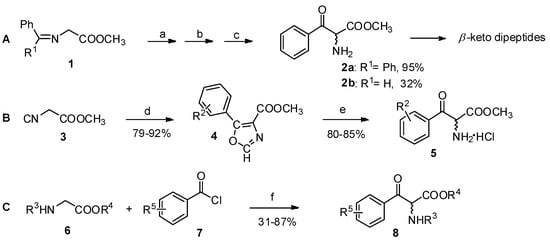
Scheme 2.
Preparation of aryl-keto α-amino acid derivatives by Claisen condensation.
Scheme 2.
Preparation of aryl-keto α-amino acid derivatives by Claisen condensation.
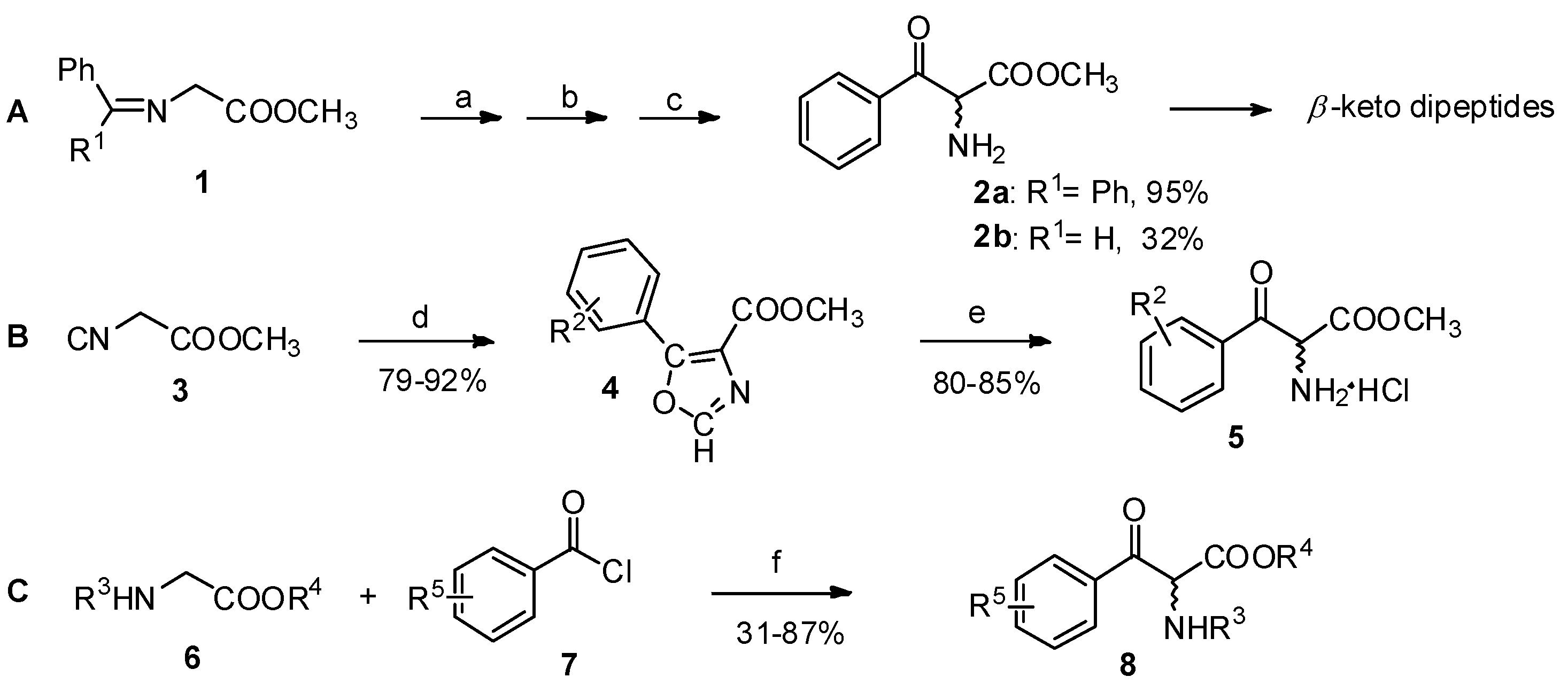
Reagents and conditions: (a) KOt-Bu, THF, −78 °C; (b) PhCOCl; (c) aq. HCl; (d) R2COCl or (R2CO)2O, 1,8-diazabicyclo-[5.4.0]undec-7-ene; (e) MeOH-HCl; (f) (i) n-BuLi, THF, −78 °C; (ii) ZnCl2, lithium diisopropylamide, −78 °C. R1 = Ph, H; R2 = 3,4-methylenedioxy-, 3,4,5-trimethoxy-, 3,4-dichloro-; R3 = Ts, TFA, Boc; R4 = t-Bu, Me, Bn; R5 = H-, 4-phenyl-, cyclohexyl-.
The cross-coupling reaction is widely used in organic synthesis to directly form a C-C bond between two components. Through the reaction of acyl or alkyl halides of α-amino acid derivatives with organic metal compounds, in the presence of palladium catalysts, numerous synthesis of aryl-keto α-amino acid derivatives have been reported (Scheme 3) [19,20].
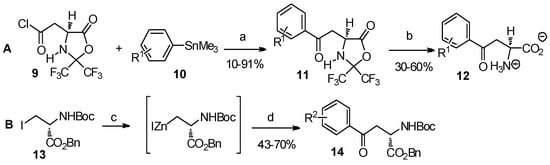
Scheme 3.
Cross-coupling reaction: a useful method to construct aryl-keto α-amino acids.
Scheme 3.
Cross-coupling reaction: a useful method to construct aryl-keto α-amino acids.
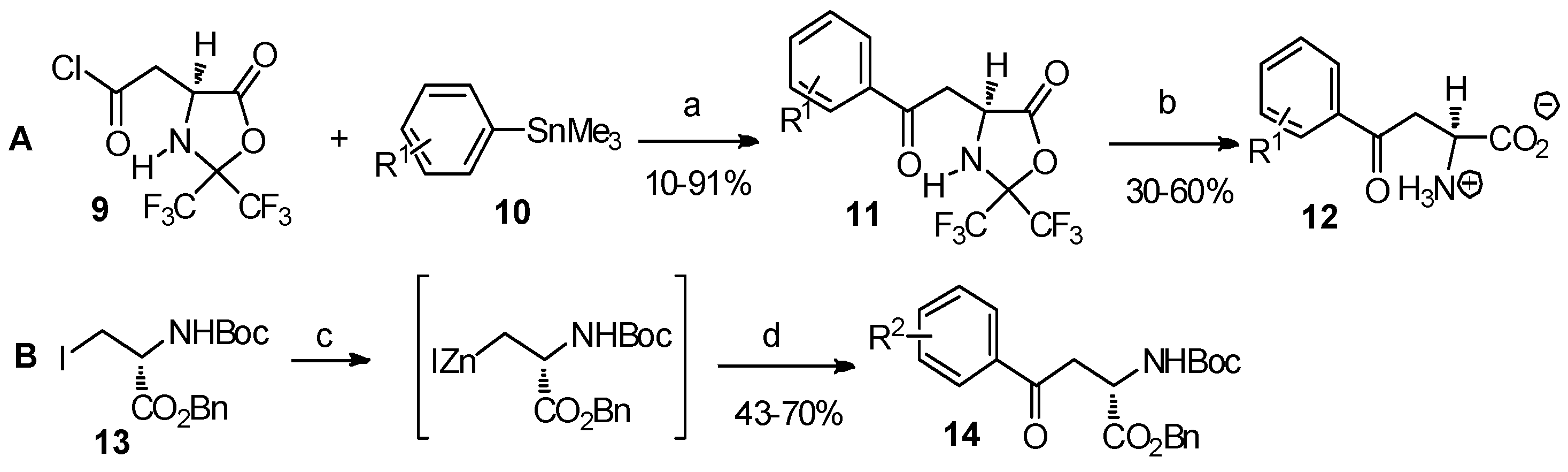
Reagents and conditions: (a) palladium catalysts: [(Pd(0): Pd2(DBA)3·CHCl3, Pd(PPh3)4; Pd(II): PhCH2Pd(PPh3)2Cl and (Ph3P)2PdCl2]. (b) H2O/i-PrOH; (c) Zn/Cu, dimethylacetamide, benzene; (d) R2COCl, (Ph3P)2PdCl2. R1 = H-, 4-fluoro-; R2 = H-, 4-methoxy-, 4-acetoxy-.
The major barriers to their application are the difficulties in the preparation of organic metal compounds. Acetamidomalonate 16 has been used for the classical synthesis of α-amino acids by C-alkylation, under basic conditions for many decades [21,22,23,24]. Through the reaction between acetamidomalonate 16 and α-bromoacetophenone (15), 4-phenyl-4-oxo-2-acetamido-2-ethoxycarbonylbutyrate (17) was easily obtained (Scheme 4) [25]. Following acid hydrolysis and enzymatic resolution using carboxypeptidase A digestion, α-benzoyl-L-alanine and α-benzoyl-D-alanine were isolated in high yield and enantiomeric purity. A more comprehensive study has been reported by Varasi and coworkers [26]. Through the reaction of acetamidomalonate and α-chlorocetophenone, a series of kynurenic acid derivatives were prepared. Meanwhile, in the presence of tert-butyl lithium, acetamidomalonate could readily react with benzoyl chloride to form the corresponding β-keto amino acid derivative [27].
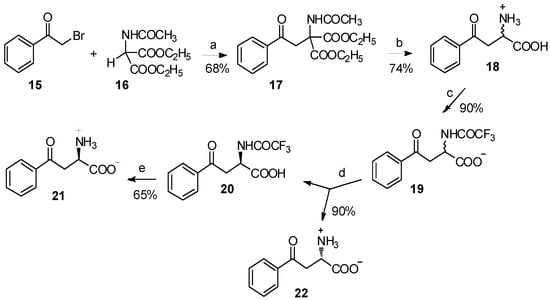
Scheme 4.
Synthesis of α-benzoyl-L-alanine and α-benzoyl-D-alanine from acetamidomalonate.
Scheme 4.
Synthesis of α-benzoyl-L-alanine and α-benzoyl-D-alanine from acetamidomalonate.
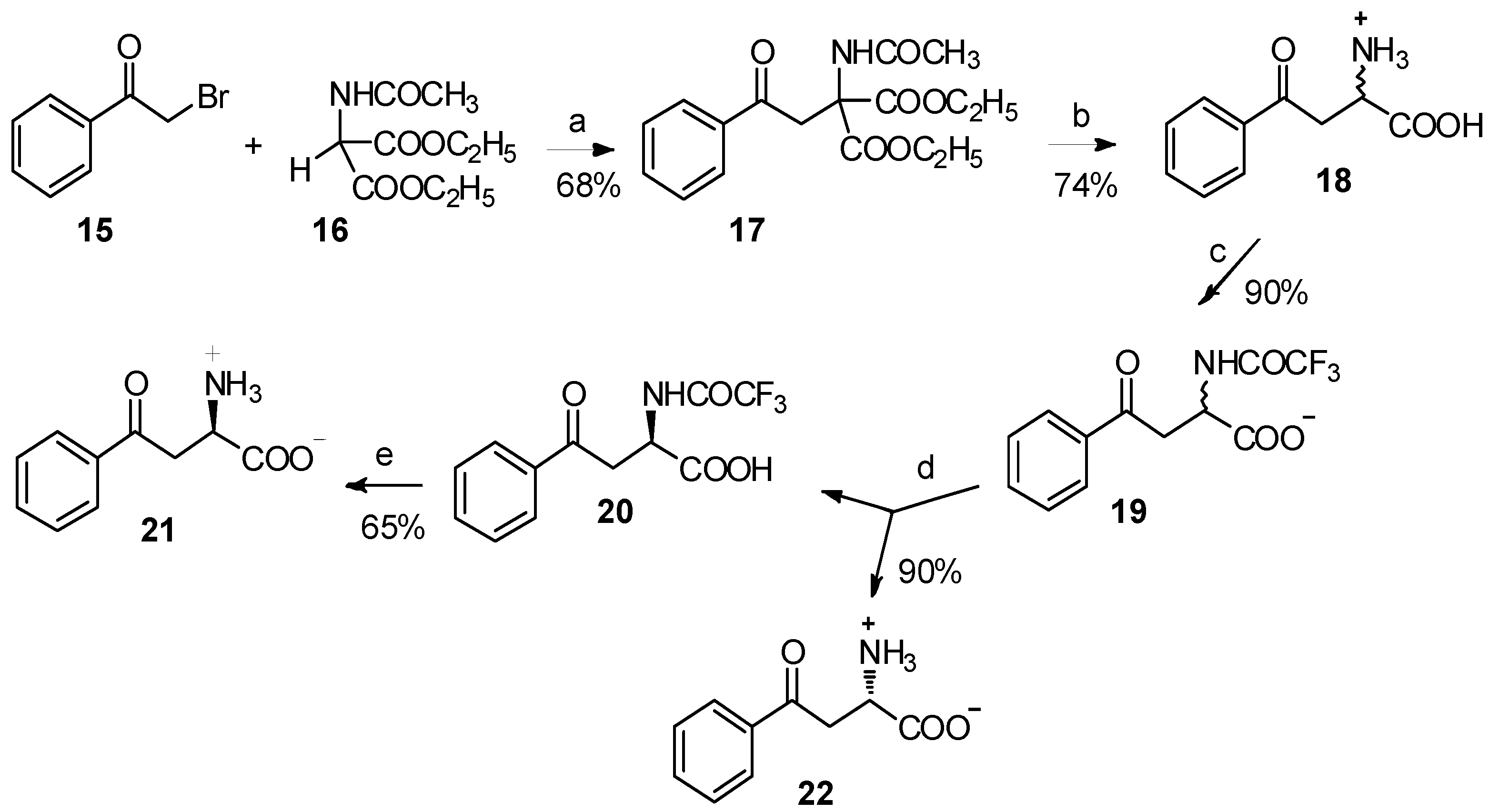
Reagents and conditions: (a) NaH, DMF, 0 °C; (b) 6 N HCl, 1,4-dioxane, reflux; (c) ethyl trifluoroacetate, tetramethylguanidine, DMF; (d) carboxypeptidase A, pH = 7.8; (e) NaOH.
In addition, the Grignard reaction of azlactones [28], nucleophilic substitution with phenyl lithium [29,30] or α-bromoalanine derivative [31], oxidation of tryptophan [32], reaction of N-acylimino acetates and trimethylsilyl enol ethers [33], acylation reaction with lithio dianion [34], reaction of Schiff base esters and silyl enol ether of acetophenone [35] are also useful methods for the preparation of aryl-keto α-amino acids and their corresponding derivatives.
2.2. Non-Amino Acid Derivatives as the Reactants
It has been reported that the nucleophilic reaction of ethyl benzoylacetate with diazonium is feasible for the preparation of ethyl α-phenylazobenzoylacetate [36], which can be used as a useful intermediate for the preparation of β-phenylserine (Scheme 5A). And the chiral amine can also be used as a useful amine-donor in Michael addition with benzoylacrylic acid to prepare aryl-keto α-amino acid derivatives (Scheme 5B) [10].
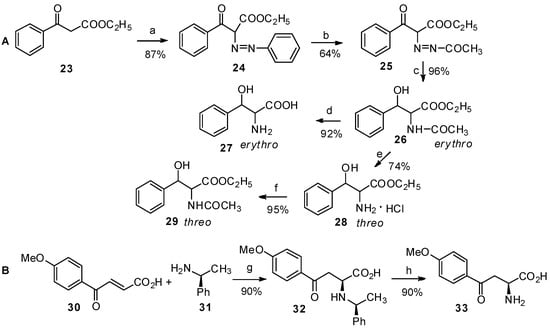
Scheme 5.
Preparation of aryl-keto α-amino acids from non-amino acid derivatives.
Scheme 5.
Preparation of aryl-keto α-amino acids from non-amino acid derivatives.
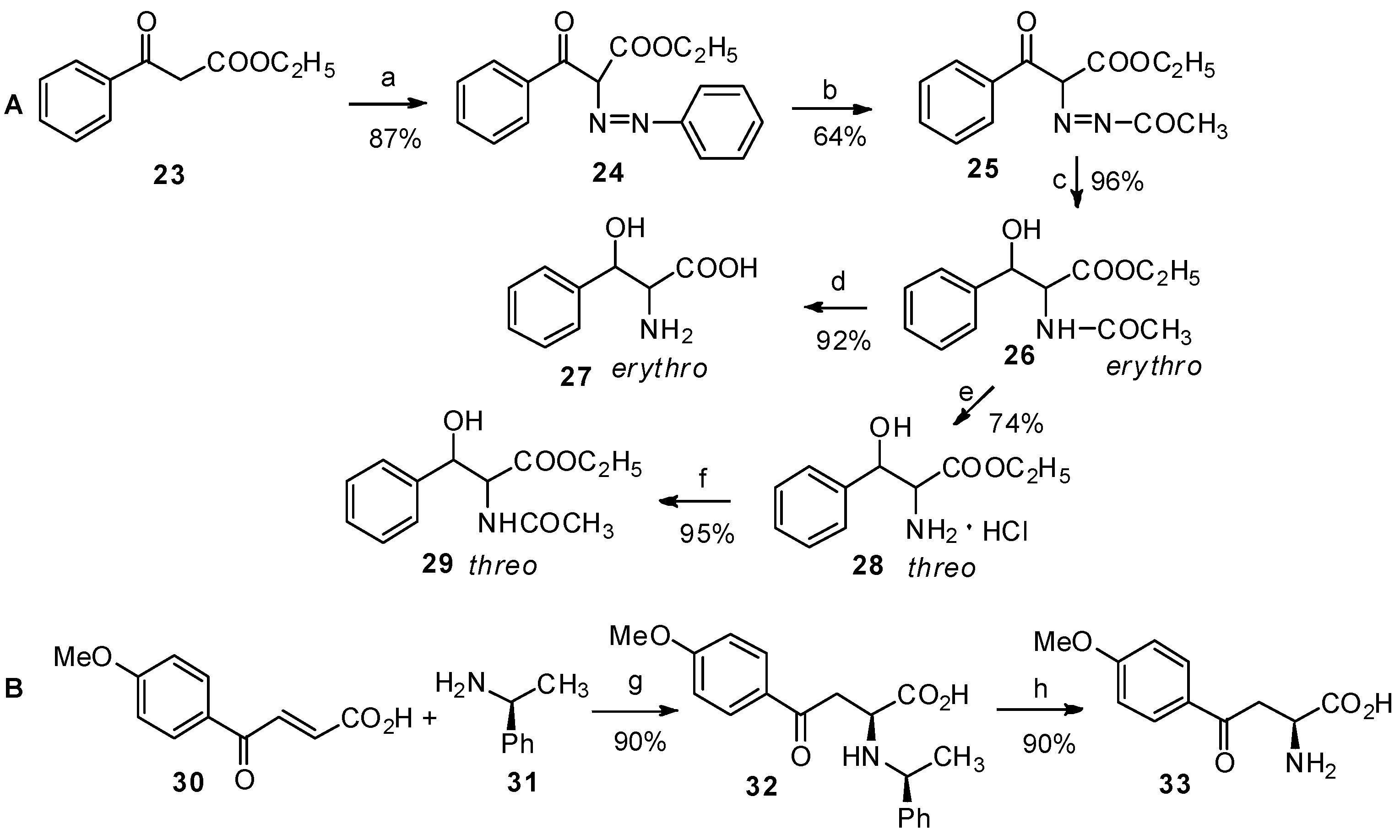
Reagents and conditions: (a) sodium nitrite, HCl, sodium acetate; (b) acetic acid, Zn, acetic anhydride; (c) H2, Pd/C, acetic acid; (d) dilute HCl, NH4OH; (e) (i) SOCl2; (ii) dilute HCl; (iii) HCl/ethanol; (f) sodium acetate, acetic anhydride: (g) methanol, 60 °C; (h) H2, Pd/C, ethanol.
Furthermore, the reaction of an organometallic serine derivative and benzaldehyde following further oxidation [37], intramolecular acyl migration reaction of acyclic imides [38], Diels-Alder reaction with amino acid dienophile [39], cross-coupling reactions with aromatic dithianes [40] are also feasible for the preparation of aryl-keto α-amino acid derivatives.
The general methods for the preparation of aryl-keto α-amino acids based on α-amino acid derivatives as the reactants or non-amino acid derivatives are of great importance. Most of these processes are sophisticated, involve cumbersome steps and the use of special catalysts, in addition to requiring time-consuming chiral resolution.
3. Preparation of Aryl Keto α-amino Acids by Friedel-Crafts Acylation
Friedel-Crafts acylation is one of the most powerful methods in organic synthesis [41], in which the Lewis acid or Brönsted acid is an indispensible catalyst to promote the reactions [42,43,44,45]. α-Amino acids such as aspartic acid and glutamic acid are crucial chiral pool reagents for the preparation of enantiomerically pure compounds. Friedel-Crafts acylation with α-amino acid derivatives as acyl donors is a convenient strategy to construct aryl keto α-amino acids with the remaining enantiomer configuration. For this purpose, numerous Friedel-Crafts acylations of stoichiometric amounts of α-amino acid derivatives were reported with AlCl3, HF, or TfOH as catalysts.
3.1. AlCl3 Catalyzed Friedel-Crafts Acylation
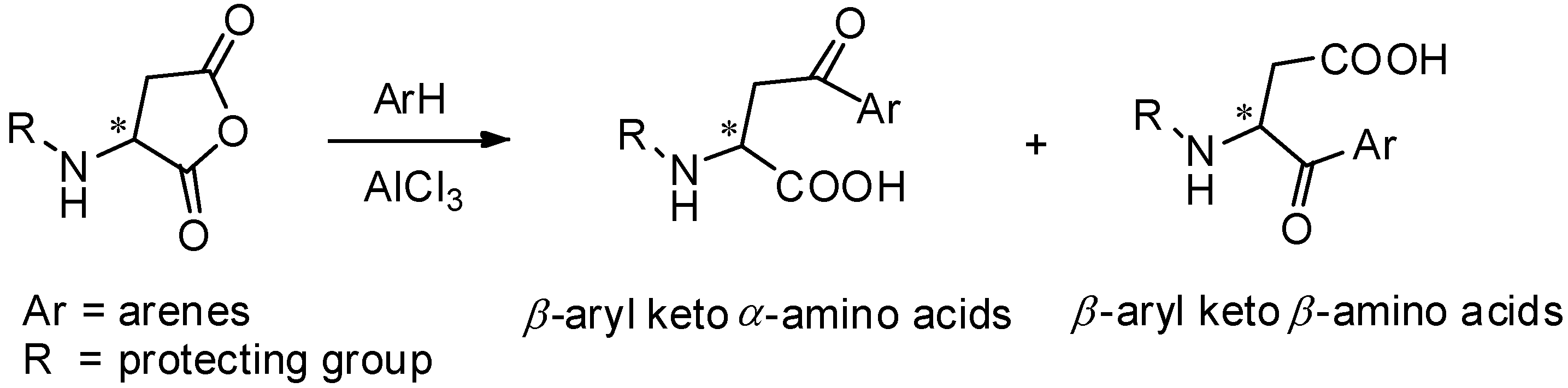
AlCl3 is the most commonly used catalyst in Friedel-Crafts acylation [46] and has been widely used for the preparation of aryl keto α-amino acids. Aspartic anhydrides, effective and popular acyl donors, are well studied for this purpose. For avoiding the racemization of optically active amino acids during the reaction, amino groups should be protected. Due to the specific structure of anhydrides, the regioselectivity should be taken into consideration. Generally, two kinds of products were obtained as β-aryl keto α-amino acids and β-aryl keto β-amino acids (Scheme 6).
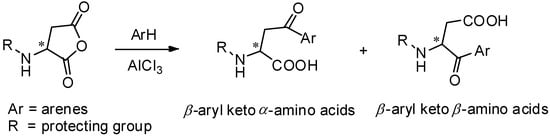
Scheme 6.
α- and β- amino acids derived from aspartic anhydrides.
Scheme 6.
α- and β- amino acids derived from aspartic anhydrides.
In 1976, Reifenrath and coworkers reported the Friedel-Crafts acylation of phthalylaspartic anhydride and benzene in the presence of AlCl3 (Scheme 7A) [47]. Through comprehensive study, they found that acylation occurred to produce β-aryl keto α-amino acids 35 as the single products. The same work was reported by Xu, along with the mechanism of the reaction (Scheme 7B) [48]. Due to the strong electron withdrawing effect of the N-phthaloylamino group to α-carbonyl group, the resonance form for generation of β-aryl keto α-amino acids 38 was more stable.
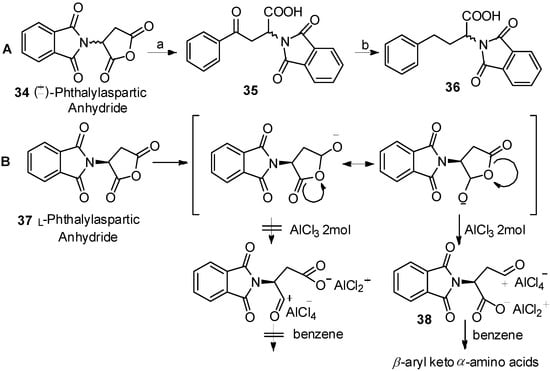
Scheme 7.
Acylation direction of phthalylaspartic anhydride in Friedel-Crafts acylation.
Scheme 7.
Acylation direction of phthalylaspartic anhydride in Friedel-Crafts acylation.
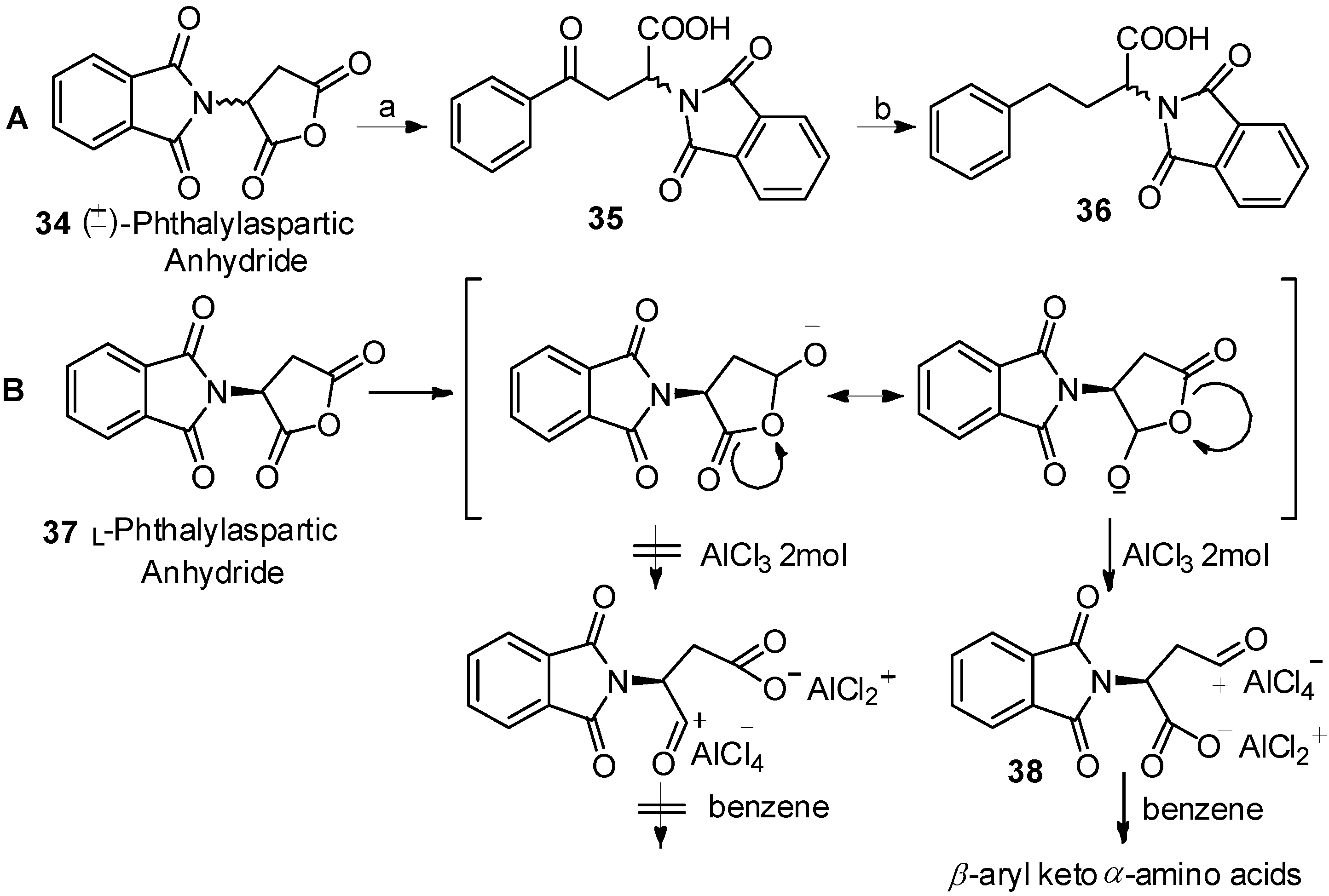
Reagents and conditions: (a) benzene, AlCl3; (b) H2/Pt.
Another study was reported by Nordlander and coworkers in 1985 (Scheme 8) [49]. N-(Trifluoroacetyl) aspartic anhydride (40) was treated with veratrole (39) to construct aryl-keto α-amino acid derivatives for the preparation of 2-amino-6,7-dihydroxy-l,2,3,4-tetrahydronaphthalene (ADTN) derivative 42, which was a powerful agonist of dopamine [50].
As part of developing a strategy for the construction of aryl-keto α-amino acids for preparation of dopamine agonists, Melillo and coworkers reported the reaction of 2-chloroanisole with N-(methoxycarbonyl) aspartic anhydride (44) resulting in the generation of single isomer 45 (Scheme 9) [13]. The key finding in this study was that chlorine as a removable directing group prevented the generation of other isomers to produce para ketone as a single product.
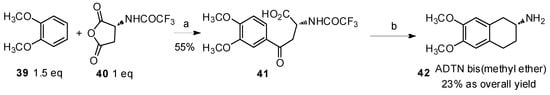
Scheme 8.
Preparation of ADTN bis(methyl ether) from veratrole by Friedel-Crafts acylation.
Scheme 8.
Preparation of ADTN bis(methyl ether) from veratrole by Friedel-Crafts acylation.
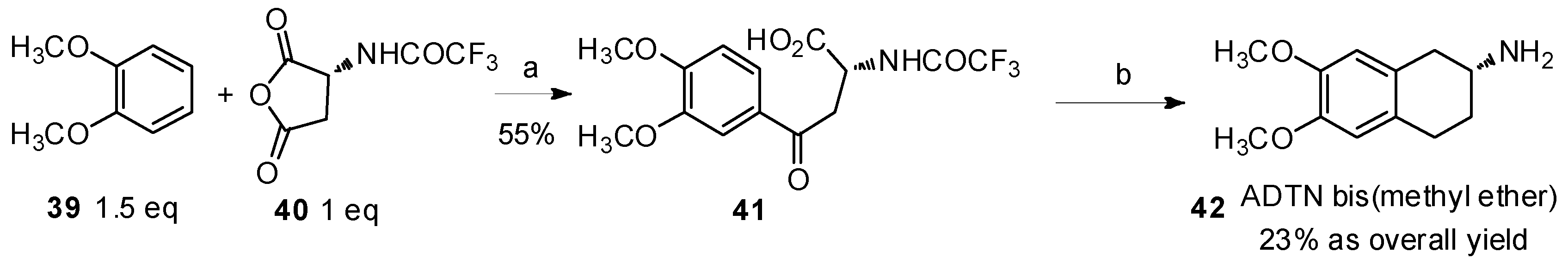
Reagents and conditions: (a) AlCl3 (2.5 eq), CH2Cl2; (b) (i) triethylsilane, trifluoroacetic acid; (ii) PCl5, CH2Cl2, SnCl4, 0 °C; (iii) triethylsilane, BF3·Et2O; (iv) K2CO3, MeOH, H2O, reflux.
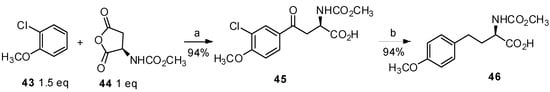
Scheme 9.
Preparation of homotyrosine derivative from 2-chloroanisole.
Scheme 9.
Preparation of homotyrosine derivative from 2-chloroanisole.
Reagents and conditions: (a) AlCl3 (2.5 eq), CH2Cl2, MeNO2; (b) H2, Pd/C, i-PrOH.
To further study the regioselectivity of anhydrides, Griesbeck and Heckroth published a comprehensive discussion about the reaction between aspartic anhydrides and a variety of aromatics [51]. Several N-protected aspartic anhydrides 47a–47e, 37 were reacted with numerous aromatics for investigation of the effect factor for α/β-selectivity of the products. As a conclusion, both the N-protecting groups and aromatics affect the α/β-selectivity of products (Table 1).

Table 1.
Regioselectivity of anhydrides in Friedel-Crafts acylation. 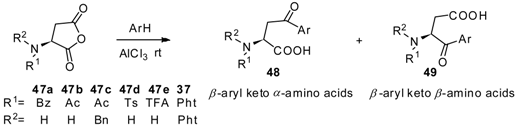

| Substrate | arene | Time (h) | α/β-amino acids ratio | Yield (%) | Substrate | arene | Time (h) | α/β-amino acids ratio | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| 47a | benzene | 5 | 55/45 | 51 | 47c | toluene | 5 | 7/93 | 47 |
| 47a | toluene | 5 | 39/61 | 33 | 47c | o-xylene | 15 | 39/61 | 52 |
| 47a | o-xylene | 15 | 30/70 | 65 | 47d | benzene | 5 | 84/16 | 76 |
| 47a | mesitylene | 15 | 100/0 | 51 | 47d | toluene | 5 | 100/0 | 71 |
| 47a | anisole | 72 | 50/50 | 31 | 47d | o-xylene | 15 | 74/26 | 83 |
| 47a | veratrole | 96 | 100/0 | 18 | 47e | benzene | 5 | 95/5 | 78 |
| 47a | naphthalene | 15 | 95/5 | 44 | 47e | toluene | 5 | 80/20 | 62 |
| 47b | benzene | 5 | 95/5 | 76 | 47e | o-xylene | 15 | 56/44 | 70 |
| 47b | toluene | 5 | 64/36 | 52 | 37 | benzene | 5 | 100/0 | 64 |
| 47b | o-xylene | 15 | 58/42 | 67 | 37 | toluene | 5 | 88/12 | 71 |
| 47c | benzene | 5 | 30/70 | 55 | 37 | o-xylene | 15 | 48/52 | 83 |
It is well known that amino-protecting groups of anhydrides not only determine the ratio of α/β-amino acids but also prevent the racemization of optically active amino acids during the reactions. The most commonly used amino-protecting groups are as follows: ethoxycarbonyl, methoxycarbonyl, trifluoroacetyl, benzoyl, tosyl, acetyl, phthaloyl groups and so on. While the preparation of N-protected amino acids is not easy due to the racemization [52], the indispensable deprotection makes large scale-production difficult. In this case, Lin and coworkers reported a precedent of α-amino acid anhydride hydrochloride as the acyl-donor in the Friedel–Crafts acylation reaction (Scheme 10) [12]. L-Aspartic anhydride hydrochloride (51), prepared from L-aspartic acid [53], was treated with anhydrous benzene in the presence of anhydrous AlCl3 to form aryl-keto α-amino acid hydrochloride 52 in high yield. Upon further hydrogenolysis, L-homophenylalanine (53), a versatile building block for the synthesis of pharmaceutical drugs such as angiotensin converting enzyme (ACE) inhibitors, β-lactam antibiotics, acetylcholinesterase inhibitors, and neutral endopeptidase inhibitors [54], was obtained in quantitative yield with >99% ee by HPLC.
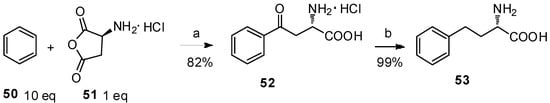
Scheme 10.
L-Aspartic anhydride hydrochloride as the acyl-donor in Friedel–Crafts acylation.
Scheme 10.
L-Aspartic anhydride hydrochloride as the acyl-donor in Friedel–Crafts acylation.
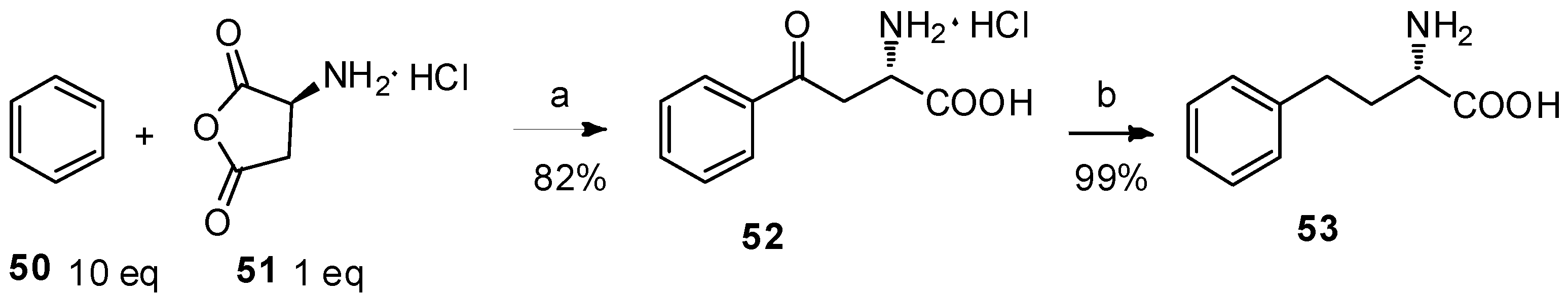
Reagents and conditions: (a) AlCl3 (2.6 eq), MeNO2, reflux; (b) H2, Pd/C, 2 N HCl, 55 °C.
Friedel-Crafts acylation catalyzed by Lewis acids leads to aryl-keto α-amino acids as well as their derivatives. However, due to the low solubility of α-amino acid derivatives in common organic solvents, reaction yields are low and sometimes a large excess of aromatics, long reaction times, and high temperatures are required. Furthermore, the environmental pollution caused by these catalysts has to be dealt with.
3.2. HF Catalyzed Friedel-Crafts Acylation
HF has been used as a catalyst and solvent in Friedel-Crafts acylation for many years [55,56]. It has also been used for the construction of aryl-keto α-amino acid derivatives. In 1998, Bednarek reported the preparation of amino acids with aryl-keto functions in the side chains [57]. In this study, ω-carboxyl-α-amino acid 54 was treated with aromatics such as anisole, 2-methoxybiphenyl, butyl phenyl ether, or 1,3-dimethoxybenzene in the presence of HF as a catalyst. All of the aryl-keto α-amino acid derivatives were obtained in high yield in the form of HF salts, which were further incorporated into peptides by conventional methods of coupling (Scheme 11).
3.3. TfOH Catalyzed Friedel-Crafts Acylation
TfOH is one of the most important Brönsted acid catalysts used in Friedel-Crafts acylation [44,45,58,59]. Due to its high dissolving capacity, it can easily dissolve most of the α-amino acid derivatives. Thus, TfOH catalyzed Friedel-Crafts acylation is a promising strategy to prepare aryl-keto α-amino acids from α-amino acid derivatives.
In recent years, our research group has been interested in the application of Friedel-Crafts acylation with TfOH as catalyst and solvent. For example, by using TfOH, an effective Friedel-Crafts acylation of O- or C-arylglucosides was carried out at low temperature for 10 min and no deglycosidation was detected [60]. Introduction of biotin to aromatics through Friedel-Crafts acylation with TfOH afforded numerous biotin derivatives, many of which showed stronger avidin binding potential than biotin [61]. More importantly, synthesis of aryl-keto α-amino acids and their derivatives by TfOH catalyzed Friedel-Crafts acylations were well studied.
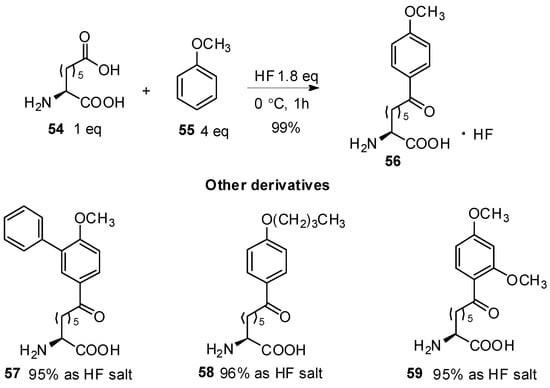
Scheme 11.
HF as catalyst in Friedel-Crafts acylation.
Scheme 11.
HF as catalyst in Friedel-Crafts acylation.
For convenient synthesis of L-homophenylalanine as well as its derivatives, aryl-keto α-amino acid derivatives were prepared as precursors from N-TFA-L-Asp(Cl)-OMe (60) with aromatics, followed by hydrogenolysis and deprotection (Table 2) [62]. N-TFA-protected L-Asp anhydride 47e, one of the most popular acyl donors as described previously, was treated with benzene in the presence of different catalysts such as AlCl3, TiCl4, H2SO4, and TfOH. The results indicated that the reaction proceeds well with TfOH in the generation of α- and β- carboxyl configuration products as a mixture. Further, another acyl-donor, L-aspartic anhydride hydrochloride (51), was treated with benzene while no product was detected when TfOH was used as a catalyst. To avoid the formation of β-carboxyl configuration products as well as improve the yield from the reaction, N-TFA-L-Asp(Cl)-OMe [63] was used as an acyl-donor to react with benzene. As expected, 98% of the α-carboxyl configuration structure was obtained as a single product within an hour, which indicated that 60 was more reactive than 47e or 51. Because 60 contained only one acylation site, the formation of the β-carboxyl configuration was prevented. Based on this result, numerous aromatics were reacted with 60 in the presence of TfOH. Through further hydrogenolysis and deprotection, L-homophenylalanine derivatives were obtained with good overall yields.
Inspired by this study, an effective synthesis of optically active trifluoromethyldiazirinyl homophenylalanine by Friedel-Crafts acylation with TfOH was reported in 2009 (Scheme 12) [64]. Trifluoromethyldiazirinyl is widely used as a photophore in photoaffinity labeling [65,66]. Upon UV irradiation, the photophore containing ligand can link to receptors or biomolecules by covalent bonds, which makes it feasible for further separation and identification. It has been reported that the diazirine ring is not stable in the presence of Lewis acid over room temperature [67], while catalytic amounts of TfOH do not affect the diazirinyl moiety [68], making it feasible for our work. In this study, N-TFA-Asp(Cl)-OMe (64) was used as an acyl-donors due to its good reactivity [62]. It was found that 3-phenyl-3-(3-trifluoromethyl)-3H-diazirine (63) did not react with N-TFA-Asp(Cl)-OMe, while higher temperature promoted the decomposition of diazirine [67]. It has been revealed that m-methoxy-substituted 3-phenyl-3-(3-trifluoromethyl)-3H-diazirine (65) not only show higher reactivity, but the methoxy group can be utilized for further introduction of the tag [65]. In this case, 3-(3-methoxyphenyl)-3-(trifluoromethyl)-3H-diazirine (65) was treated with N-TFA-Asp(Cl)-OMe (64) in the presence of TfOH at 0 °C and the desired products were obtained in good yield as expected. Due to the decomposition of the N-N double bond under traditional H2-Pd/C conditions [69], the carbonyl group was reduced by the triethylsilane/TFA system and through further deprotection, the desired products were obtained in high yield without racemization. Furthermore, the photolysis properties of these diazirinyl compounds were examined and appropriate values were obtained.

Table 2.
Friedel–Crafts reaction of anhydrides and stoichiometric amounts of benzene. 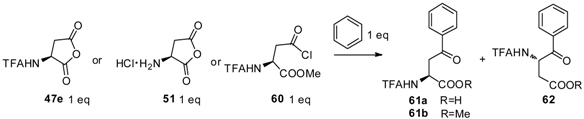

| Entry | Donor | Catalyst (eq) | Solvent | Reaction Time (h) | Product Yiled (%) |
|---|---|---|---|---|---|
| 1 | 47e | AlCl3 (8) | CH2Cl2 | 1 or 12 | 0 |
| 2 | 47e | TiCl4 (90) | neat | 1 or 12 | 1 |
| 3 | 47e | H2SO4 (90) | neat | 1 or 12 | 2 |
| 4 | 47e | TfOH (40) | neat | 1 | 61a (52), 62 (3) |
| 5 | 47e | Tf2OH (25) | neat | 1 or 12 | 0 |
| 6 | 51 | TfOH (40) | neat | 1 | 0 |
| 7 | 60 | AlCl3 (8) | CH2Cl2 | 10 | 61b (50) |
| 8 | 60 | TiCl4 (90) | neat | 1 or 12 | 0 |
| 9 | 60 | H2SO4 (90) | neat | 1 or 12 | 0 |
| 10 | 60 | TfOH (40) | neat | 1 | 61b (98) |
| 11 | 60 | Tf2OH (25) | neat | 1 or 12 | 0 |
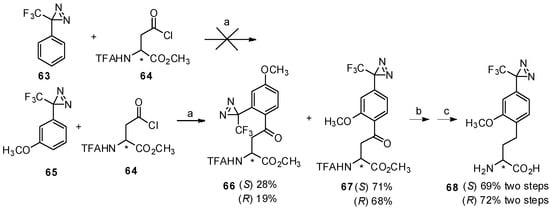
Scheme 12.
Synthesis of trifluoromethyldiazirinyl homophenylalanine by Friedel-Crafts acylation.
Scheme 12.
Synthesis of trifluoromethyldiazirinyl homophenylalanine by Friedel-Crafts acylation.
Reagents and conditions: (a) TfOH, 0 °C, 2 h; (b) triethylsilane, TFA; (c) NaOH, MeOH.
Homotyrosine has been found in many biological products [70] and its derivatives play an important role in the synthesis of natural products [71]. For effective synthesis of homotyrosines, our group reported the Friedel-Crafts acylation of N-TFA-Asp(Cl)-OMe (64) with phenol (69) in the presence of TfOH (Scheme 13) [72]. As expected, p- and o- aroylbenzene derivatives were obtained in high yield within one hour in neat TfOH. Interestingly, N-TFA-Asp-OMe β-phenyl ester (70) as an O-acylated product could be obtained from N-TFA-Asp(Cl)-OMe with phenol by using diluted TfOH, which can be reconverted to p- and o-aroylbenzene derivatives upon Fries rearrangement.
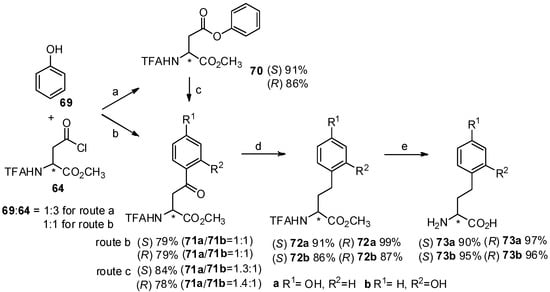
Scheme 13.
Synthesis of homotyrosines by Friedel-Crafts acylation with TfOH.
Scheme 13.
Synthesis of homotyrosines by Friedel-Crafts acylation with TfOH.
Reagents and conditions: (a) 3% TfOH-CH3CN, rt, 1 h; (b) TfOH, rt, 1 h; (c) TfOH, rt, 16 h; (d) H2, Pd/C, rt; (e) 6 N HCl, 80 °C, 6 h.
Further investigation indicated that the reaction time was longer than that of direct Friedel-Crafts acylation with neat TfOH because partial hydrolysis of phenyl ester ocurred, followed by Friedel-Crafts acylation of the carboxylic acid and aromatic hydrocarbon. Finally, two aryl-keto α-amino acid derivatives were subjected to further hydrogenolysis and deprotection to form optically pure homotyrosine derivatives.
To further extend the scope of this strategy, glutamic acid was also investigated as the other acyl-donor in the Friedel-Crafts acylation with aromatics (Scheme 14) [14]. Three aromatics (compounds 50, 74 and 55) can readily react with N-TFA-Glu(Cl)-OMe (75) in the presence of TfOH with generation of aryl-keto α-amino acids in high yield. Through further reduction and deprotection, corresponding bishomophenylalanines 78a-c [73] were obtained in high yields without any loss of chiral purity. To study other applications, 3-(3-methoxyphenyl)-3-(3-trifluoromethyl)-3H-diazirine (65) as the aryl-donor was treated with N-TFA-Glu(Cl)-OMe (75) in the presence of TfOH. As expected, stereocontrolled aryl-keto α-amino acids were obtained, which was consistent with previous reports [64]. Following reduction by the triethylsilane/TFA system and deprotection under alkaline conditions, the diazirinyl moiety-based bishomophenylalanine was obtained in high yield and optical purity. These can be used as important components in photoaffinity labeling [74].
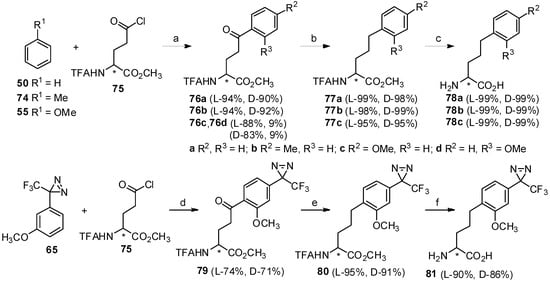
Scheme 14.
Synthesis of bishomophenylalanine and diazirinyl derivative by Friedel-Crafts acylation.
Scheme 14.
Synthesis of bishomophenylalanine and diazirinyl derivative by Friedel-Crafts acylation.
Reagents and conditions: (a) TfOH, rt, 1 h; (b) H2, Pd/C, AcOH, rt, 1 h; (c) 6 N HCl, 80 °C, 6 h; (d) TfOH, 0 °C, 3 h; (e) Et3SiH, TFA, rt, 1 h; (f) NaOH, MeOH, rt, 2 h.
It is well known that bishomotyrosine is found in the active components of AM-toxin Ⅲ [75] and its convenient synthesis is of great importance. A novel and convenient synthesis of bishomotyrosine through construction of its aryl-keto α-amino acid derivative by Friedel-Crafts acylation was reported (Scheme 15) [76]. The corresponding bishomotyrosines were obtained in high optical purity and yield.
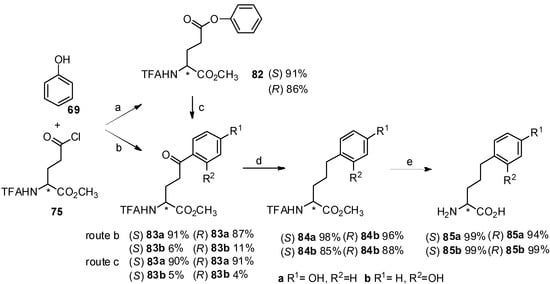
Scheme 15.
Synthesis of bishomotyrosine by Friedel-Crafts acylation with TfOH.
Scheme 15.
Synthesis of bishomotyrosine by Friedel-Crafts acylation with TfOH.
Reagents and conditions: (a) 5% TfOH-CH3CN, rt, 0.5 h; (b) TfOH, rt, 1 h; (c) TfOH, rt, 2 h; (d) H2, Pd/C, rt; (e) 6 N HCl, 80 °C, 6 h
Benzophenone is another photophore that is widely used in photoaffinity labeling [77,78]. The benzophenone-based α-amino acids are promising structures for protein study. In 1986, Kaure J. C. and co-workers were the first to report the synthesis of p-benzoyl-L-phenylalanine (91) as a new benzophenone-based amino acid analog (Scheme 16) [79]. Furthermore, p-methylbenzoic acid, followed by bromination and treatment with Schiff’s base-activated glycine under basic and phase-transfer conditions, can also be used to prepare p-benzoyl-L-phenylalanine derivatives [80]. But there are no reports that benzophenone skeleton was constructed on phenylalanine with Friedel-Crafts benzoylation due to the solubility of phenylalanine in organic solvents.
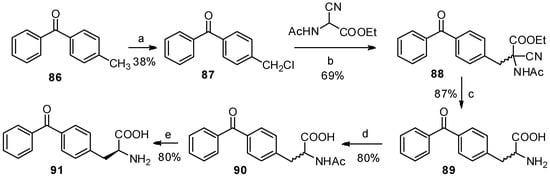
Scheme 16.
Previous synthesis of p-benzoyl-L-phenylalanine from p-methylbenzophenone.
Scheme 16.
Previous synthesis of p-benzoyl-L-phenylalanine from p-methylbenzophenone.
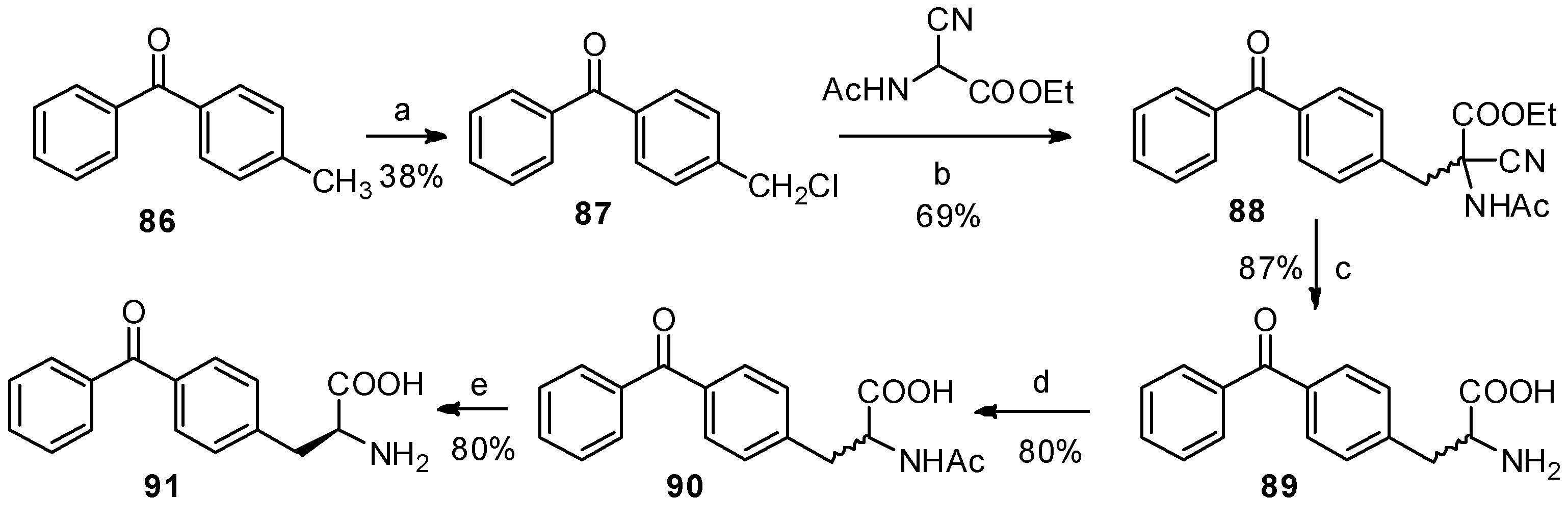
Reagents and conditions: (a) dibenzoyl peroxide, SO2Cl2; (b) K2CO3, acetone; (c) (i) 8 N HCl; (ii) hot water, 1 N NaOH, (d) acetic anhydride, NaOH; (e) (i) NH4OH, aspergillus acylase I, toluene; (ii) 5 N HCl, 70 °C, Celite; (iii) 1 N NaOH.
For convenient synthesis of benzophenone-based α-amino acid derivatives, we carried out Friedel-Crafts acylation between phenylalanine derivatives 92a-b and diazirinylbenzoyl chloride 95 in the presence of TfOH [81]. Furthermore, two photophore (benzophenone and diazirine) based structures (97) were prepared as an interesting study and their detailed analysis of photo-irradiation were also investigated, which may contribute to the investigation of peptide-receptor interactions in photoaffinity labeling (Scheme 17).
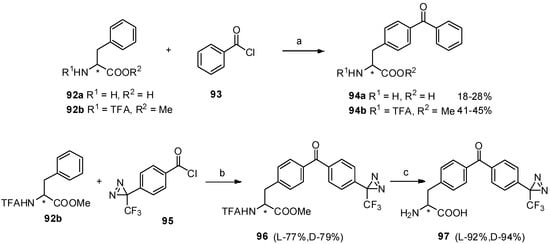
Scheme 17.
Synthesis of benzophenone based α-amino acid derivatives and two photophores based structures by Friedel-Crafts acylation with TfOH.
Scheme 17.
Synthesis of benzophenone based α-amino acid derivatives and two photophores based structures by Friedel-Crafts acylation with TfOH.
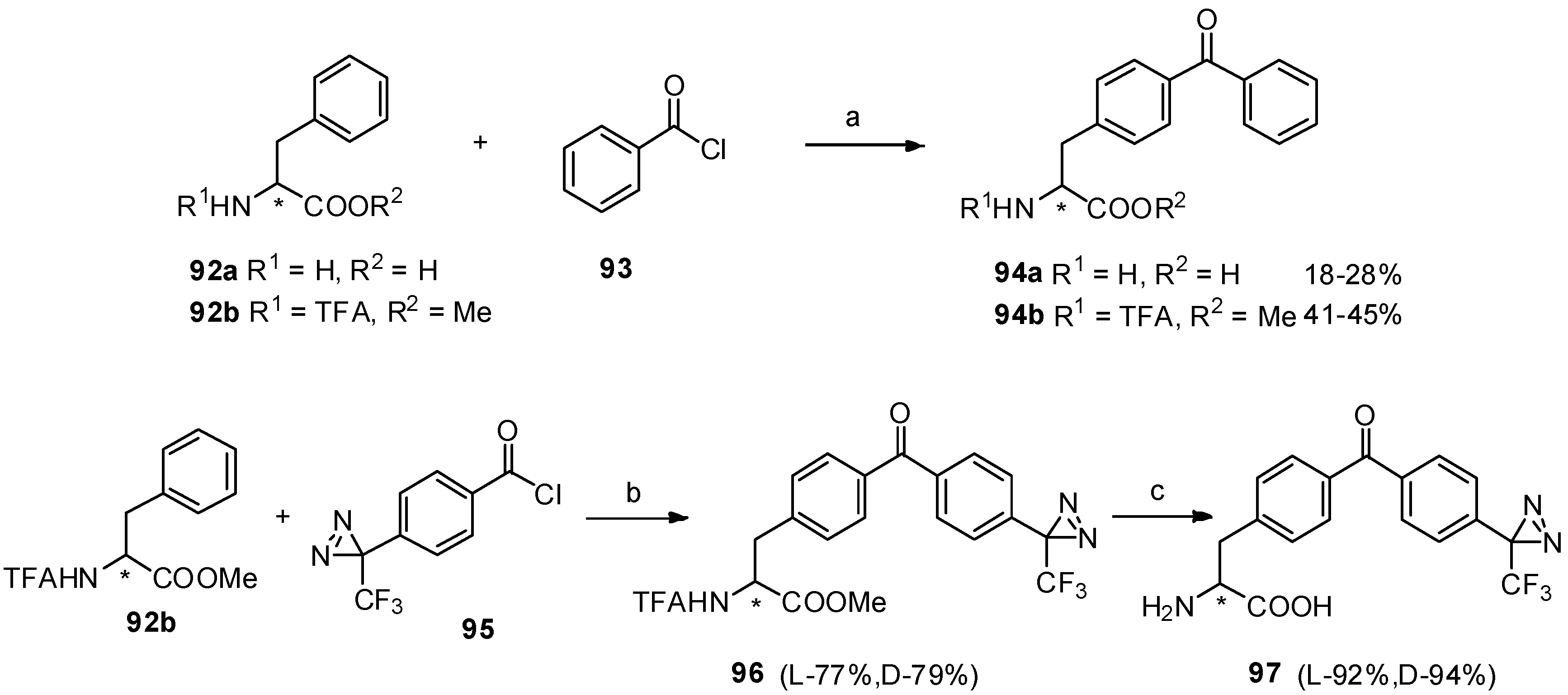
Reagents and conditions: (a) TfOH; (b) TfOH, 0 °C; (c) NaOH.
TfOH is not only an excellent solvent but also an effective catalyst in the Friedel-Crafts acylation of α-amino acid derivatives and aromatics. Inspired by these results, we investigated the utilization of TfOD for this study, to ensure the status of the generated acylation (Scheme 18) [82]. N-TFA-L-Glu(Cl)-OMe (98) was treated with toluene in the presence of TfOD at room temperature for 1 h, and aryl-keto α-amino acid derivatives were obtained in high yield. Interestingly, it was found that hydrogen/deuterium exchange occurred on the aromatic ring and the deuterium incorporation was calculated to be 68%–75% based on 1H-NMR spectroscopic analysis.
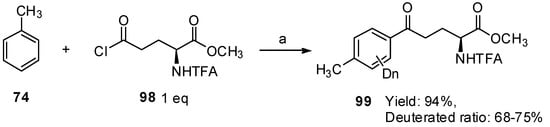
Scheme 18.
Friedel–Crafts reaction and hydrogen/deuterium exchange with TfOD.
Scheme 18.
Friedel–Crafts reaction and hydrogen/deuterium exchange with TfOD.
Reagents and conditions: (a) TfOD, rt, 1 h.
In comparison with TfOH, TfOD performs the same role in Friedel-Crafts acylation between α-amino acid derivatives and aromatics. On the other hand, TfOD is also the deuterium-donating reagent, which effectively promotes hydrogen/deuterium exchange on aromatic α-amino acids. As a part of developing a novel strategy, a series of hydrogen/deuterium exchanges for aromatic α-amino acids and their corresponding peptides were also performed in the presence of TfOD. Furthermore, TfOD-catalyzed hydrogen/deuterium exchange was also carried out on some cross-linkable α-amino acid derivatives, which will contribute to effective analysis of biological functions of bioactive peptides and proteins by MS [83].
4. Conclusions
In conclusion, aryl-keto α-amino acid derivatives, excellent building blocks in organic chemistry and biochemistry, can be readily prepared under different conditions using several strategies. Among them, Friedel-Crafts acylation in the presence of AlCl3, HF, or TfOH(D) is one of the most convenient and effective methods. As a strong Brönsted acid, TfOH behaves not only as a catalyst, but also as an excellent solvent for dissolving α-amino acid derivatives that are not easy to dissolve in common organic solvents. Following further hydrogenolysis and deprotection, a lot of useful non-proteinogenic amino acids and functional structures such as photophores containing α-amino acid derivatives are obtained with high optical purity and yield.
Acknowledgments
This research was partially supported by the Fugaku Foundation, Noastec Foundation and the Cooperative Research Program of “Network Joint Research Center for Materials and Devices” for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hartung, W.H.; Dittrich, T.T.; Chang, Y.T. β-Arylserine ethyl esters. J. Am. Chem. Soc. 1953, 75, 238–239. [Google Scholar] [CrossRef]
- Hernández, D.; Vilar, G.; Riego, E.; Cañedo, L.M.; Cuevas, C.; Albericio, F.; Álvarez, M. Synthesis of IB-01211, a cyclic peptide containing 2,4-concatenated thia- and oxazoles, via Hantzsch macrocyclization. Org. Lett. 2007, 9, 809–811. [Google Scholar]
- Ojika, M.; Inukai, Y.; Kito, Y.; Hirata, M.; Iizuka, T.; Fudou, R. Miuraenamides: Antimicrobial cyclic depsipeptides isolated from a rare and slightly halophilic myxobacterium. Chem. Asian J. 2008, 3, 126–133. [Google Scholar] [CrossRef]
- Vanderhaeghe, H.; Parmentier, G. The structure of factor S of staphylomycin. J. Am. Chem. Soc. 1960, 82, 4414–4422. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yamaki, H.; Shinoda, T.; Tago, Y.; Suzuki, H.; Nishimura, T.; Yamaguchi, H. The mode of antifungal action of (S)2-amino-4-oxo-5-hydroxypentanoic acid, RI-331. J. Antibiot. 1990, 43, 411–416. [Google Scholar] [CrossRef]
- Kumar, S.; Gawandi, V.B.; Capito, N.; Phillips, R.S. Substituent effects on the reaction of β-benzoylalanines with Pseudomonas fluorescens kynureninase. Biochemistry 2010, 49, 7913–7919. [Google Scholar] [CrossRef]
- Weiβ, T.D.; Helmchen, G.; Kazmaier, U. Synthesis of amino acid derivatives via enantio- and diastereoselective Pd-catalyzed allylic substitutions with a non-stabilized enolate as nucleophile. Chem. Commun. 2002, 2002, 1270–1271. [Google Scholar]
- Arend, M. Asymmetric catalytic aminoalkylations: New powerful methods for the enantioselective synthesis of amino acid derivatives, Mannich bases, and homoallylic amines. Angew. Chem. Int. Ed. 1999, 38, 2873–2874. [Google Scholar] [CrossRef]
- Ferraris, D.; Young, B.; Dudding, T.; Lectka, T. Catalytic, enantioselective alkylation of α-imino esters using late transition metal phosphine complexes as catalysts. J. Am. Chem. Soc. 1998, 120, 4548–4549. [Google Scholar] [CrossRef]
- Yamada, M.; Nagashima, N.; Hasegawa, J.; Takahashi, S. A highly efficient asymmetric synthesis of methoxyhomophenylalanine using Michael addition of phenethylamine. Tetrahedron Lett. 1998, 39, 9019–9022. [Google Scholar] [CrossRef]
- Gore, P.H. The Friedel-Crafts acylation reaction and its application to polycyclic aromatic hydrocarbons. Chem. Rev. 1955, 55, 229–281. [Google Scholar] [CrossRef]
- Lin, W.; He, Z.; Zhang, H.; Zhang, X.; Mi, A.; Jiang, Y. Amino acid anhydride hydrochlorides as acylating agents in Friedel-Crafts reaction: A practical synthesis of L-homophenylalanine. Synthesis 2001, 1007–1009. [Google Scholar]
- Melillo, D.G.; Larsen, R.D.; Mathre, D.J.; Shukis, W.F.; Wood, A.W.; Colleluori, J.R. Practical enantioselective synthesis of a homotyrosine derivative and (R,R)-4-propyl-9-hydroxynaphthoxazine, a potent dopamine agonist. J. Org. Chem. 1987, 52, 5143–5150. [Google Scholar] [CrossRef]
- Murai, Y.; Hatanaka, Y.; Kanaoka, Y.; Hashimoto, M. Effective synthesis of optically active 3-phenyl-3-(3-trifluoromethyl)diazirinyl bishomophenylalanine derivatives. Heterocycles 2009, 79, 359–364. [Google Scholar] [CrossRef]
- Huckin, S.N.; Weiler, L. Claisen condensation of the dianion of β-keto esters. Tetrahedron Lett. 1972, 13, 2405–2408. [Google Scholar] [CrossRef]
- Singh, J.; Gordon, T.D.; Earley, W.G.; Morgan, B.A. An efficient synthesis and acylation of α-amino-β-keto-esters: Versatile intermediates in the synthesis of peptide mimetics. Tetrahedron Lett. 1993, 34, 211–214. [Google Scholar] [CrossRef]
- Suzuki, M.; Iwasaki, T.; Miyoshi, M.; Okumura, K.; Matsumoto, K. New convenient synthesis of α-C-acylamino acids and α-amino ketones. J. Org. Chem. 1973, 38, 3571–3575. [Google Scholar] [CrossRef]
- Schultz, K.; Stief, L.; Kazmaier, U. A straightforward approach towards α-amino-β-keto esters via acylation of chelated amino acid ester enolates. Synthesis 2012, 600–604. [Google Scholar]
- Golubev, A.S.; Sewald, N.; Burger, K. Synthesis of γ-oxo α-amino acids from L-aspartic acid. Tetrahedron 1996, 52, 14757–14776. [Google Scholar] [CrossRef]
- Jackson, R.F.W.; Wishart, N.; Wood, A.; James, K.; Wythes, M.J. Preparation of enantiomerically pure protected 4-oxo α-amino acids and 3-aryl α-amino acids from serine. J. Org. Chem. 1992, 57, 3397–3404. [Google Scholar] [CrossRef]
- Haudegond, J.P.; Chauvin, Y.; Commereuc, D. Synthesis of α-amino acids by alkylation of diethyl acetamidomalonate in the presence of palladium complexes. J. Org. Chem. 1979, 44, 3063–3065. [Google Scholar] [CrossRef]
- Leanna, M.R.; Morton, H.E. N-(Boc)-L-(2-Bromoallyl)-glycine: A versatile intermediate for the synthesis of optically active unnatural amino acids. Tetrahedron Lett. 1993, 34, 4485–4488. [Google Scholar]
- Kotha, S.; Singh, K. N-Alkylation of diethyl acetamidomalonate: Synthesis of constrained amino acid derivatives by ring-closing metathesis. Tetrahedron Lett. 2004, 45, 9607–9610. [Google Scholar] [CrossRef]
- Watanabe, L.A.; Jose, B.; Kato, T.; Nishino, N.; Yoshida, M. Synthesis of L-α-amino-ω-bromoalkanoic acid for side chain modification. Tetrahedron Lett. 2004, 45, 491–494. [Google Scholar] [CrossRef]
- Gawandi, V.B.; Liskey, D.; Lima, S.; Phillips, R.S. Reaction of Pseudomonas fluorescens kynureninase with β-benzoyl-L-alanine: Detection of a new reaction intermediate and a change in rate-determining step. Biochemistry 2004, 43, 3230–3237. [Google Scholar] [CrossRef]
- Varasi, M.; Torre, A.D.; Heidempergher, F.; Pevarello, P.; Speciale, C.; Guidetti, P.; Wells, D.R.; Schwarcz, R. Derivatives of kynurenine as inhibitors of rat brain kynurenine aminotransferase. Eur. J. Med. Chem. 1996, 31, 11–21. [Google Scholar] [CrossRef]
- Schmidt, U.; Griesser, H.; Lieberknecht, A.; Schmidt, J.; Gräther, T. Synthesis of α-acylamino-β-oxo acid esters. Synthesis 1993, 1993, 765–766. [Google Scholar] [CrossRef]
- Pines, S.H.; Karady, S.; Sletzinger, M. 3-Aryl-2-methylserines. I. A new synthesis. J. Org. Chem. 1968, 33, 1758–1761. [Google Scholar] [CrossRef]
- Berrée, F.; Chang, K.; Cobas, A.; Rapoport, H. Synthesis of anisolylated aspartyl and glutamyl tripeptides. J. Org. Chem. 1996, 61, 715–721. [Google Scholar]
- Thomas, H.A.; Ling, N.; Wei, E.T.; Berree, F.; Cobas, A.; Rapoport, H. Novel anti-inflammatory undecapeptides that contain anisolylated glutamic acid derivatives. J. Pharmacol. Exp. Ther. 1993, 267, 1321–1326. [Google Scholar]
- Münster, P.; Steglich, W. Synthesis of α-amino acids by reaction of t-butyl N-(t-butoxycarbonyl)iminoacetate with C-nucleophiles. Synthesis 1987, 1987, 223–225. [Google Scholar] [CrossRef]
- Kleijn, L.H.J.; Müskens, F.M.; Oppedijk, S.F.; Bruin, G.D.; Martin, N.I. A concise preparation of the non-proteinogenic amino acid L-kynurenine. Tetrahedron Lett. 2012, 53, 6430–6432. [Google Scholar] [CrossRef]
- Bretschneider, T.; Miltz, W.; Münster, P.; Steglich, W. New syntheses of α-amino acids based on N-acylimino acetates. Tetrahedron 1988, 44, 5403–5414. [Google Scholar] [CrossRef]
- Harding, K.E.; Moreno, L.N.; Nace, V.M. Hydroxyalkylation and acylation reactions of methyl hippurate. J. Org. Chem. 1981, 46, 2809–2812. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Bennett, W.D. The synthesis of amino acids by reaction of an electrophilic glycine cation equivalent with neutral carbon nucleophiles. Tetrahedron 1988, 44, 5389–5401. [Google Scholar] [CrossRef]
- Bolhofer, W.A. β-Phenylserine. J. Am. Chem. Soc. 1952, 74, 5459–5461. [Google Scholar] [CrossRef]
- Barfoot, C.W.; Harvey, J.E.; Kenworthy, M.N.; Kilburn, J.P.; Ahmed, M.; Taylor, R.J.K. Highly functionalised organolithium and organoboron reagents for the preparation of enantiomerically pure α-amino acids. Tetrahedron 2005, 61, 3403–3417. [Google Scholar] [CrossRef]
- Hara, O.; Ito, M.; Hamada, Y. Novel N → C acyl migration reaction of acyclic imides: A facile method for α-aminoketones and β-aminoalcohols. Tetrahedron Lett. 1998, 39, 5537–5540. [Google Scholar] [CrossRef]
- Olsen, R.K.; Feng, X. Synthesis of oxazolidine derivatives of β-[3-(aryloxy)aryl]-α-amino acids by application of the Diels-Alder reaction. Tetrahedron Lett. 1991, 32, 5721–5724. [Google Scholar] [CrossRef]
- Chacko, S.; Ramapanicker, R. Synthesis of γ-oxo γ-aryl and γ-aryl α-amino acids from aromatic aldehydes and serine. Eur. J. Org. Chem. 2012, 2012, 7120–7128. [Google Scholar] [CrossRef]
- Price, C.C. Alkylation and Related Reactions. In Friedel-Crafts and Related Reactions; Olah, G.A., Ed.; Interscience (Wiley): New York, NY, USA, 1964; volume 145, pp. 1174–1175. [Google Scholar]
- Kangani, C.O.; Day, B.W. Mild, efficient Friedel−Crafts acylations from carboxylic acids using cyanuric chloride and AlCl3. Org. Lett. 2008, 10, 2645–2648. [Google Scholar] [CrossRef]
- Bensari, A.; Zaveri, N.T. Titanium(IV) chloride-mediated ortho-acylation of phenols and naphthols. Synthesis 2003, 2003, 267–271. [Google Scholar]
- Anderson, K.W.; Tepe, J.J. Trifluoromethanesulfonic acid catalyzed Friedel–Crafts acylation of aromatics with β-lactams. Tetrahedron 2002, 58, 8475–8481. [Google Scholar] [CrossRef]
- Sarvari, M.H.; Sharghi, H. Simple and improved procedure for the regioselective acylation of aromatic ethers with carboxylic acids on the surface of graphite in the presence of methanesulfonic acid. Synthesis 2004, 2165–2168. [Google Scholar]
- Cook, D. The interaction of Friedel-Crafts catalysts with organic molecules I. The CH3COCl:AlCl3 system. Can. J. Chem. 1959, 37, 48–53. [Google Scholar] [CrossRef]
- Reifenrath, W.G.; Bertelli, D.J.; Micklus, M.J.; Fries, D.S. Stereochemistry of Friedel-Crafts addition of phthalylaspartic anhydride to benzene. Tetrahedron Lett. 1976, 17, 1959–1962. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, G.; Wang, X.; Wu, T.; Pan, X.; Chan, A.S.C.; Yang, T.K. The synthesis of l-(+)-homophenylalanine hydrochloride. Tetrahedron-Asymmetry 2000, 11, 2309–2314. [Google Scholar] [CrossRef]
- Nordlander, J.E.; Payne, M.J.; Njoroge, F.G.; Vishwanath, V.M.; Han, G.R.; Laikos, G.D.; Balk, M.A. A short enantiospecific synthesis of 2-amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene (ADTN). J. Org. Chem. 1985, 50, 3619–3622. [Google Scholar] [CrossRef]
- Horn, A.S.; Kazemier, H.G.; Dijkstra, D. Brain levels and metabolism of the dopaminergic agonist 2-amino-6,7-dihydroxytetrahydronaphthalene after administration of various prodrugs. J. Med. Chem. 1982, 25, 993–996. [Google Scholar]
- Griesbeck, A.G.; Heckroth, H. A simple approach to β-amino acids by acylation of arenes with N-acyl aspartic anhydrides. Synlett 1997, 1997, 1243–1244. [Google Scholar] [CrossRef]
- Buckley, T.F., III; Rapoport, H. α-Amino acids as chiral educts for asymmetric products. Amino acylation with N-acylamino acids. J. Am. Chem. Soc. 1981, 103, 6157–6163. [Google Scholar] [CrossRef]
- Ariyoshi, Y.; Yamatani, T.; Uchiyama, N.; Sato, N. The convenient preparation of L-aspartic anhydride hydrochloride and hydrobromide. Bull. Chem. Soc. Jpn. 1972, 45, 2208–2209. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Oh, P.C.; Shukor, S.R.A. Sustainable biocatalytic synthesis of l-homophenylalanine as pharmaceutical drug precursor. Biotechnol. Adv. 2009, 27, 286–296. [Google Scholar] [CrossRef]
- Wiechert, K. Neuere Methoden der präparativen organischen Chemie II 2. Verwendung von Fluorwasserstoff für organisch-chemische Reaktionen. Angew. Chem. 1943, 56, 333–342. (In German) [Google Scholar] [CrossRef]
- Baasner, B.; Klauke, E. Reactions in anhydrous hydrogen fluoride. J. Fluor. Chem. 1982, 19, 553–564. [Google Scholar] [CrossRef]
- Bednaiek, M.A. Amino acids with aryl-keto function in their side chains. J. Peptide Res. 1998, 52, 195–200. [Google Scholar] [CrossRef]
- Effenberger, F.; Epple, G. Catalytic Friedel-Crafts acylation of aromatic compounds. Angew. Chem. Int. Ed. Engl. 1972, 11, 300–301. [Google Scholar]
- Hwang, J.P.; Prakash, G.K.S.; Olah, G.A. Trifluoromethanesulfonic acid catalyzed novel Friedel–Crafts acylation of aromatics with methyl benzoate. Tetrahedron 2000, 56, 7199–7203. [Google Scholar] [CrossRef]
- Hashimoto, M.; Takahashi, M. Effective Friedel-Crafts acylations of O- and C-arylglycosides with triflic acid. Heterocycles 2009, 77, 227–231. [Google Scholar] [CrossRef]
- Muto, Y.; Murai, Y.; Sakihama, Y.; Hashidoko, Y.; Hashimoto, M. Effective Friedel-Crafts acylation of biotin acid chloride in trifluoromethanesulfonic acid. Biosci. Biotechnol. Biochem. 2012, 76, 2162–2164. [Google Scholar] [CrossRef]
- Murashige, R.; Hayashi, Y.; Hashimoto, M. Asymmetric and efficient synthesis of homophenylalanine derivatives via Friedel–Crafts reaction with trifluoromethanesulfonic acid. Tetrahedron Lett. 2008, 49, 6566–6568. [Google Scholar] [CrossRef]
- Svete, J.; Stanovnik, B.; Tiŝler, M. The synthesis of azatryptophane derivatives. J. Heterocycl. Chem. 1994, 31, 1259–1266. [Google Scholar] [CrossRef]
- Murashige, R.; Murai, Y.; Hatanaka, Y.; Hashimoto, M. Effective synthesis of optically active trifluoromethyldiazirinyl homophenylalanine and aroylalanine derivatives with the Friedel-Crafts reaction in triflic acid. Biosci. Biotechnol. Biochem. 2009, 73, 1377–1380. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hatanaka, Y. Recent progress in diazirine-based photoaffinity labeling. Eur. J. Org. Chem. 2008, 2008, 2513–2523. [Google Scholar] [CrossRef]
- Dubinsky, L.; Krom, B.P.; Meijler, M.M. Diazirine based photoaffinity labeling. Bioorg. Med. Chem. 2012, 20, 554–570. [Google Scholar] [CrossRef]
- Moss, R.A.; Fedé, J.M.; Yan, S. SbF5-mediated reactions of oxafluorodiazirines. Org. Lett. 2001, 3, 2305–2308. [Google Scholar] [CrossRef]
- Nakashima, H.; Hashimoto, M.; Sadakane, Y.; Tomohiro, T.; Hatanaka, Y. Simple and versatile method for tagging phenyldiazirine photophores. J. Am. Chem. Soc. 2006, 128, 15092–15093. [Google Scholar] [CrossRef]
- Ambroise, Y.; Mioskowski, C.; Djéga-Mariadassou, G.; Rousseau, B. Consequences of affinity in heterogeneous catalytic reactions: Highly chemoselective hydrogenolysis of iodoarenes. J. Org. Chem. 2000, 65, 7183–7186. [Google Scholar] [CrossRef]
- Okumura, H.S.; Philmus, B.; Portmann, C.; Hemscheidt, T.K. Homotyrosine-containing cyanopeptolins 880 and 960 and anabaenopeptins 908 and 915 from Planktothrix agardhii CYA 126/8. J. Nat. Prod. 2008, 72, 172–176. [Google Scholar]
- Canesi, S.; Bouchu, D.; Ciufolini, M.A. Fully stereocontrolled total syntheses of (−)-cylindricine C and (−)-2-epicylindricine C: A departure in sulfonamide chemistry. Angew. Chem. Int. Ed. 2004, 43, 4336–4338. [Google Scholar] [CrossRef]
- Murashige, R.; Hayashi, Y.; Ohmori, S.; Torii, A.; Aizu, Y.; Muto, Y.; Murai, Y.; Oda, Y.; Hashimoto, M. Comparisons of O-acylation and Friedel–Crafts acylation of phenols and acyl chlorides and Fries rearrangement of phenyl esters in trifluoromethanesulfonic acid: Effective synthesis of optically active homotyrosines. Tetrahedron 2011, 67, 641–649. [Google Scholar] [CrossRef]
- Plaza, A.; Bewley, C.A. Largamides A−H, unusual cyclic peptides from the marine cyanobacterium Oscillatoria sp. J. Org. Chem. 2006, 71, 6898–6907. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yoshioka, T.; Morita, C.; Sakai, M.; Okuno, T.; Shirahama, H. Synthesis of a photoaffinity-labeling analog of alternariolide (AM-toxin I), a host-specific phytotoxin. Chem. Lett. 1998, 27, 203–204. [Google Scholar]
- Ueno, T.; Nakashima, T.; Hayashi, Y.; Fukami, H. Isolation and structure of AM-toxin III, a host specific phytotoxic metabolite produced by Alternaria mali. Agr. Biol. Chem. 1975, 39, 2081–2082. [Google Scholar] [CrossRef]
- Murai, Y.; Hashidoko, Y.; Hashimoto, M. Novel synthesis of optically active bishomotyrosine derivatives using the Friedel-Crafts reaction in triflic acid. Biosci. Biotechnol. Biochem. 2011, 75, 352–354. [Google Scholar] [CrossRef]
- Wang, J.; Bauman, S.; Colman, R.F. Photoaffinity labeling of rat liver glutathione S-transferase, 4-4, by glutathionyl S-[4-(succinimidyl)-benzophenone]. Biochemistry 1998, 37, 15671–15679. [Google Scholar] [CrossRef]
- Abe, I.; Zheng, Y.; Prestwich, G.D. Photoaffinity labeling of oxidosqualene cyclase and squalene cyclase by a benzophenone-containing inhibitor. Biochemistry 1998, 37, 5779–5784. [Google Scholar] [CrossRef]
- Kauer, J.C.; Erickson-Viitanen, S.; Wolfe, H.R.; DeGrado, W.F. p-Benzoyl-L-phenylalanine, a new photoreactive amino acid. J. Biol. Chem. 1986, 261, 10695–10700. [Google Scholar]
- Dorman, G.; Olszewski, J.D.; Prestwich, G.D. Synthesis of highly tritiated 4-benzoyl-L-phenylalanine, a photoactivatable amino acid. J. Org. Chem. 1995, 60, 2292–2297. [Google Scholar] [CrossRef]
- Murai, Y.; Wang, L.; Muto, Y.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Simple and stereocontrolled preparation of benzoylated phenylalanine using Friedel–Crafts reaction in trifluoromethanesulfonic acid for photoaffinity labeling. Heterocycles 2013, 87, 2119–2126. [Google Scholar] [CrossRef]
- Murai, Y.; Wang, L.; Masuda, K.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Rapid and controllable hydrogen/deuterium exchange on aromatic rings of α-amino acids and peptides. Eur. J. Org. Chem. 2013, 2013, 5111–5116. [Google Scholar] [CrossRef]
- Wang, L.; Murai, Y.; Yoshida, T.; Okamoto, M.; Masuda, K.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Hydrogen-deuterium exchange of cross-linkable α-amino acid derivatives in deuterated triflic acid. Biosci. Biotechnol. Biochem. in press. 2014; in press. [Google Scholar]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
