Abstract
Perilla (Perilla frutescens L.) leaves have shown therapeutic efficacy in the treatment of inflammatory disorders, allergies, bronchial asthma, and systemic damage due to free radicals. In the present study we analyzed the active constituents in perilla leaves using high-performance liquid chromatography (HPLC) and isolated luteolin, a polyphenolic flavonoid. We investigated the anti-inflammatory and antipruritic properties of luteolin. Luteolin inhibited the secretion of inflammatory cytokines such as interleukin-1β (IL-1 β) and tumor necrosis factor-α (TNF-α) from human mast cells (HMC-1) stimulated with phorbol myristate acetate plus calcium ionophore A23187 in a dose-dependent manner. Luteolin also significantly reduced the histamine release from rat peritoneal mast cells stimulated by compound 48/80, a potent histamine liberator. Furthermore, the administration of luteolin markedly inhibited the scratching behavior and vascular permeability induced by pruritogens, such as compound 48/80 or serotonin, in ICR mice. These results suggested that luteolin has potential as a therapeutic agent against inflammation and itch-related skin diseases.
Keywords:
perilla leaves; luteolin; mast cells; scratching behavior; anti-inflammation; antipruritus 1. Introduction
Inflammation is a protective aspect of the body’s response to injury or infection and induces release of various chemicals that stimulate nerve endings. The main symptoms of inflammation include redness, swelling, heat and pain. In chronic inflammatory diseases caused by persistent inflammation, a progressive shift of the cell type at the area of inflammation occurs, causing considerable tissue damage [1]. During inflammation, the inflammatory region is infiltrated with mononuclear cells such as monocytes, macrophages and lymphocytes, producing a wide range of inflammatory mediators including pro-inflammatory cytokines [2].
Mast cells have been recognized for their participation in allergic reactions as well as in inflammatory processes based on their ability to secrete numerous cytokines, chemokines and preformed mediators such as histamine [3,4]. When mast cells are activated by allergens bound to IgE-FcεRI on the cell surface, they release histamine and mast cell granule proteins as well as a wide variety of pro-inflammatory mediators such as interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor (TNF)-α, which may exacerbate allergic diseases [5,6]. These mediators affect nerve, muscle, and endothelial cells and induce itching, contraction, vasodilation and edema. Notably, modulation of mast cell cytokine production can provide a useful therapeutic strategy for allergic inflammatory diseases, which include atopic dermatitis, allergic asthma and allergic rhinitis [7].
Pruritus or itch, which is defined as an unpleasant cutaneous sensation associated with the immediate desire to scratch, is the diagnostic hallmark of dermatological diseases such as atopic dermatitis [8,9]. Itch can lead to repetitive scratching until bleeding. The severe scratching causes skin lesions, and consequently aggravates the skin disease. Therefore, inhibition of the itch that provokes scratching would improve the patient’s quality of life and enable treatment of the original disease [10]. Numerous studies have focused on identification of compounds in plant extracts capable of treating itch-related skin diseases [11].
Perilla (Perilla frutescens L.) is an annual herb of the Lamiaceae family mainly used in Korea, Japan and China and with many beneficial properties [12]. Perilla leaves have long been used to treat various diseases, including depression, tumors, bacterial and fungal infections, allergies and some intestinal disorders. They also inhibit Th2-cytokine production, anti-DNP IgE production and IgA nephropathy [13,14]. The main active constituents of perilla leaves include flavonoids, saponins, polysaccharides, amino acids and trace elements [12,14,15]. The flavonoids existing in many types of plant have been reported to exert anti-inflammatory and anti-allergic activities by suppressing the proliferation and activity of lymphocytes [16,17].
In the present study we focused on luteolin, a flavonoid extracted from perilla leaves and investigated its anti-inflammatory and antipruritic effects on human mast cells (HMC-1), rat peritoneal mast cells (RPMCs) and ICR mice.
2. Results and Discussion
2.1. The Chemical Structure and Effect of Luteolin on Cell Survival
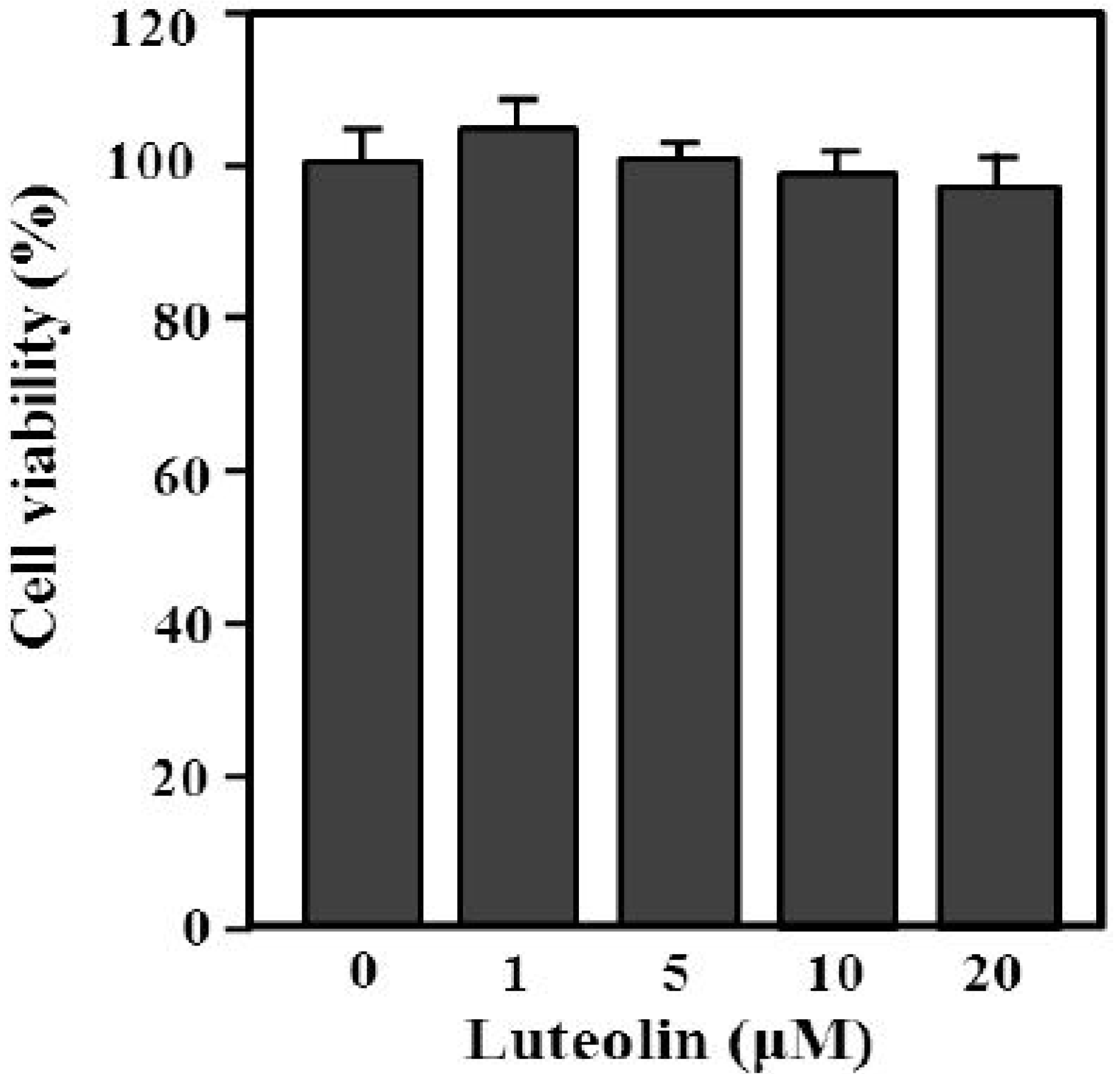
The content of luteolin isolated from perilla leaves by HPLC was 22.58 μg/g. The chemical structure of luteolin, a plant flavonoid, is shown in Figure 1. Luteolin is a pharmacologically active agent isolated from several herbal species such as fruits, vegetables and medicinal plants [18,19]. Flavonoids play an important role in protecting plant cells from microorganisms, insects and ultraviolet (UV) radiation. They also exhibit cancer preventive, antioxidative, anti-allergic and anti-inflammatory activities in cells [10,20]. Recently, luteolin isolated from the Lonicera japonica flowers was shown to inhibit pro-inflammatory cytokine and inflammatory mediator production by HMC-1 cells activated with PMA plus A23187, and suppress inflammation-associated gene expression by blocking the NF-κB pathway [21,22,23]. These reports showed that plants rich in luteolin have been used to treat diseases such as inflammation, allergy and cancer since ancient times. However, studies of the anti-inflammatory effects of luteolin isolated from perilla leaves are lacking. Therefore, we examined whether luteolin isolated from perilla leaves has anti-inflammatory and antipruritic effects in HMC-1 cells, RPMCs, and ICR mice. First, we examined the cytotoxicity of luteolin against HMC-1 cells by MTT assay. As shown in Figure 2, luteolin did not affect cell viability.
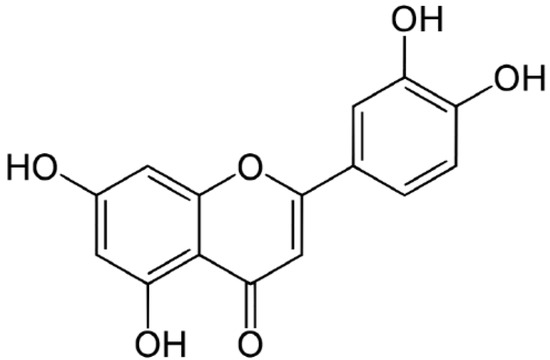
Figure 1.
The structure of luteolin isolated from perilla (P. frutescens) leaves.
Figure 1.
The structure of luteolin isolated from perilla (P. frutescens) leaves.
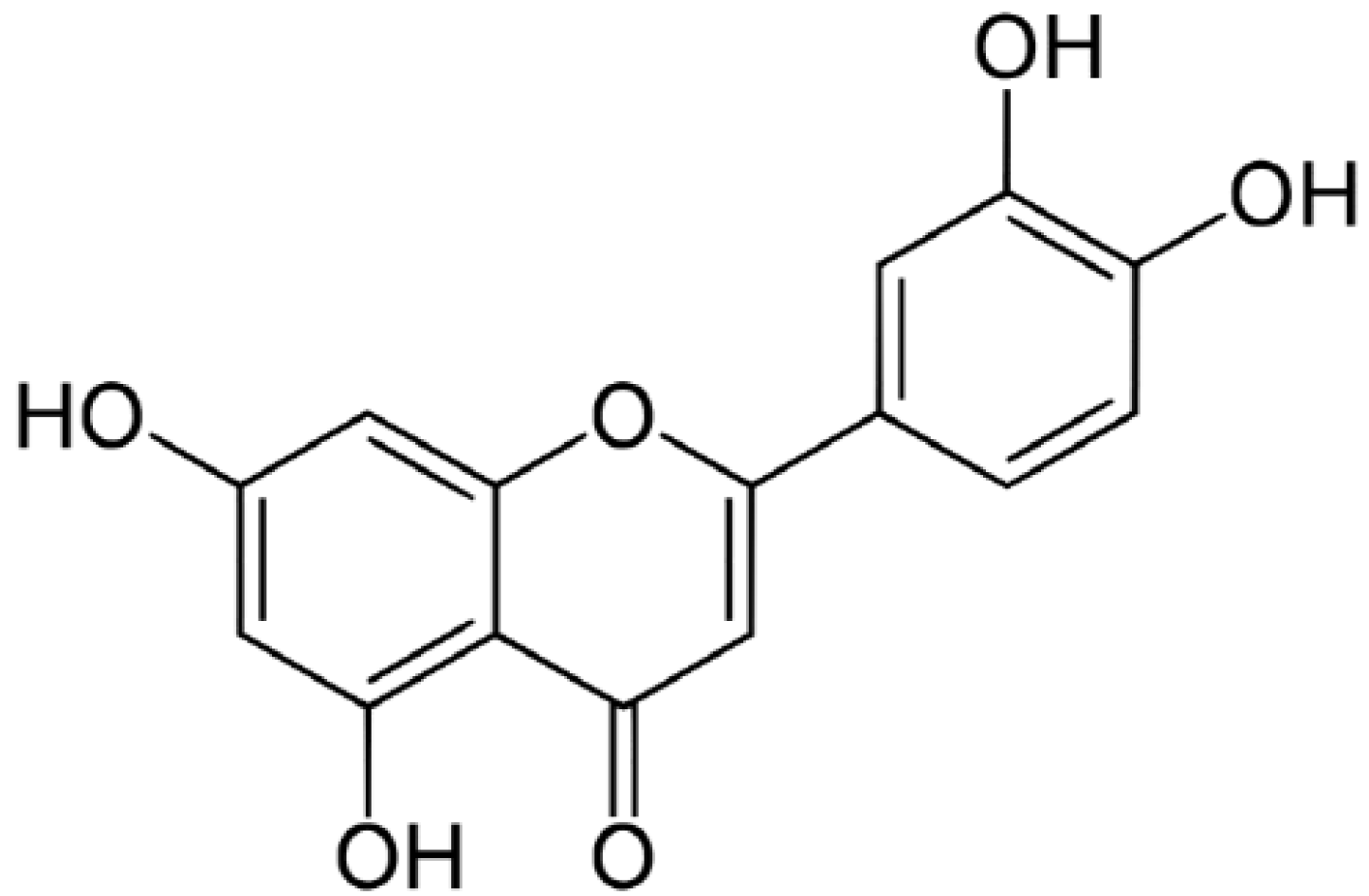
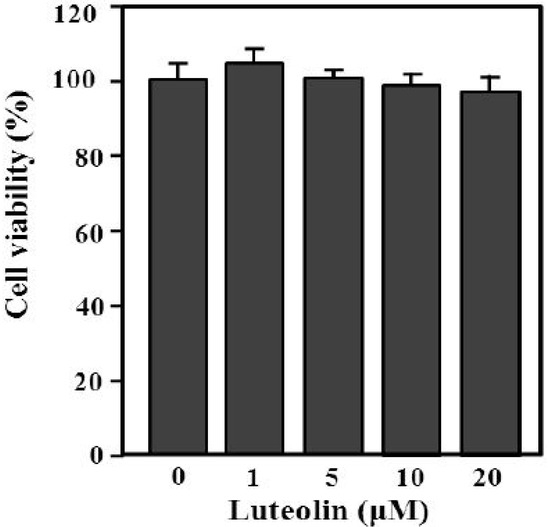
Figure 2.
Effect of luteolin on HMC-1 cell viability. Cells (5 × 105) were treated with 1–20 μM luteolin and then incubated at 37 °C for 12 h. Cell viability was determined by MTT assay. Values are means ± standard deviation (SD) of duplicate determinations from three independent experiments.
Figure 2.
Effect of luteolin on HMC-1 cell viability. Cells (5 × 105) were treated with 1–20 μM luteolin and then incubated at 37 °C for 12 h. Cell viability was determined by MTT assay. Values are means ± standard deviation (SD) of duplicate determinations from three independent experiments.
2.2. Inhibition of TNF-α and IL-1β Production by Luteolin
Mast cells are one of the major immune effector cells. Activated mast cells release pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, and inflammatory mediators, including histamine, leukotrienes, serotonin, prostaglandin (PG)E2, and PGD2 [5,7,24]. TNF-α and IL-1β cytokines are released in a coordinated network and play an important role in chronic inflammation. TNF-α is preformed and stored in granules of mast cells or newly synthesized following mast cell activation; it is a multifunctional cytokine and an important mediator of the immune and inflammatory responses. TNF-α is an autocrine stimulator as well as a potent inducer of other inflammatory cytokines, including IL-1β, IL-6, and IL-8 [6]. IL-1β is a pro-inflammatory cytokine and a potent mediator of inflammatory processes [25].
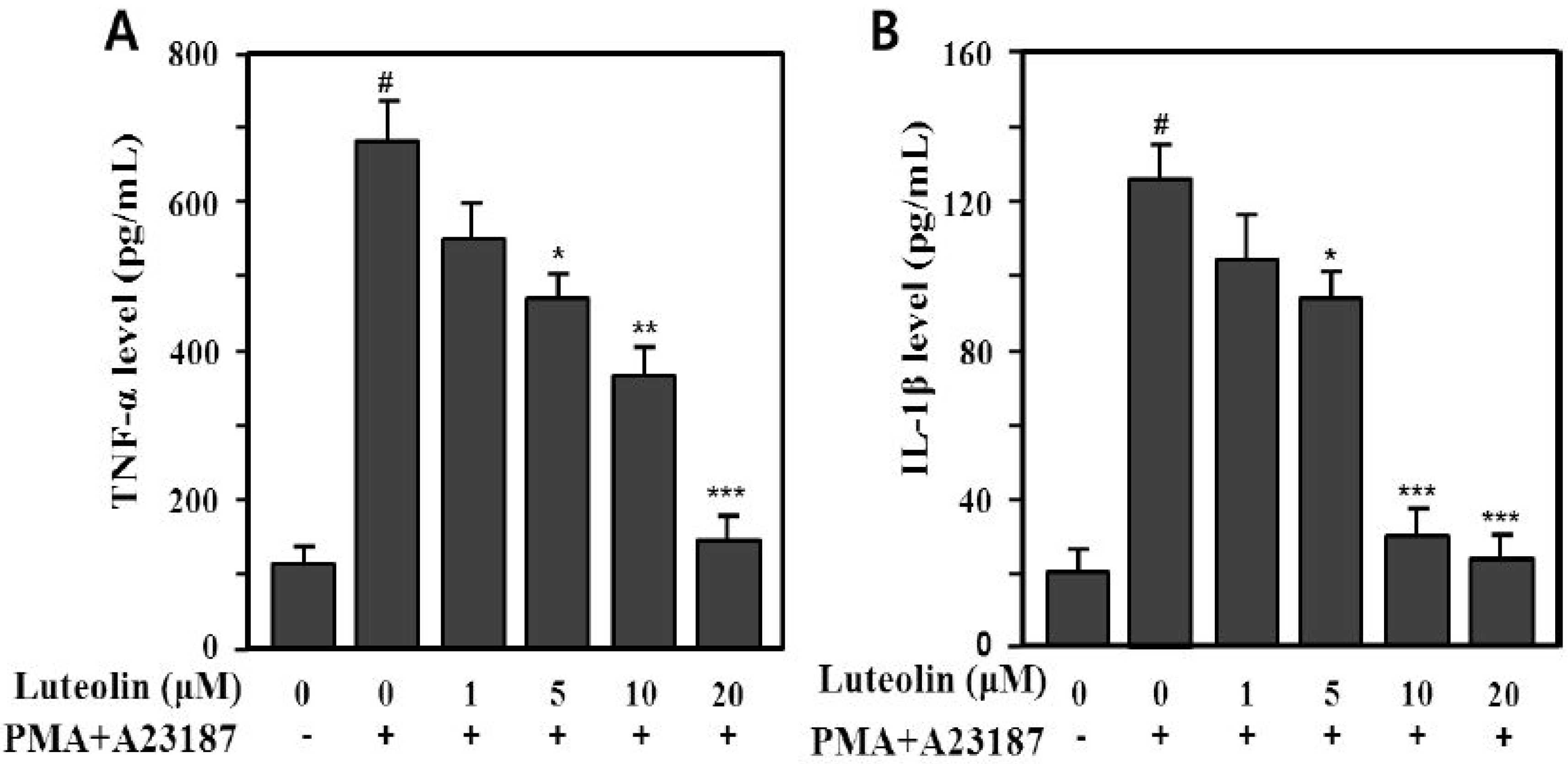
Therefore, we examined whether luteolin could modulate production of pro-inflammatory cytokines, such as TNF-α and IL-1β, induced by PMA plus A23187 in HMC-1 cells. The supernatants were analyzed using an enzyme-linked immunosorbent assay (ELISA) for TNF-α and IL-1β. PMA plus A23187 significantly increased TNF-α (688.5 ± 47.3 pg/mL) and IL-1β (129.2 ± 9.1 pg/mL) production compared with the medium control (TNF-α: 110.5 ± 21.3 pg/mL, IL-1β: 19.8 ± 5.11 pg/mL) in HMC-1 cells (p < 0.001). We also assessed the effect of luteolin (1–20 μM) on PMA plus A23187-induced TNF-α and IL-1β production. The increased production of TNF-α and IL-1β induced by PMA plus A23187 was significantly inhibited by 5–20 μM luteolin (Figure 3). The inhibition rates were 31.9%–76.8% (TNF-α) and 27.3%–81.2% (IL-1β) at 5–20 μM luteolin.
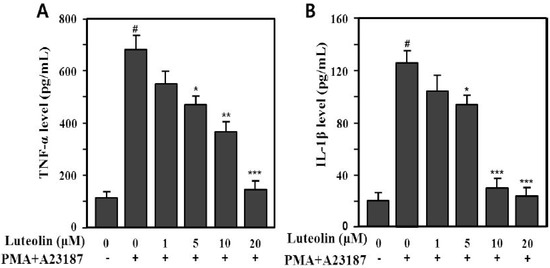
Figure 3.
Inhibitory effect of luteolin on PMA plus A23187-induced TNF-α (A)- and IL-1β (B) production in HMC-1 cells. Cells (5 × 105) were pretreated with 1–20 μM luteolin for 30 min prior to stimulation with or without 25 nM PMA plus 1 μM A23187 and then incubated at 37 °C for 12 h. Cytokine levels were determined by enzyme-linked immunosorbent assay (ELISA). Values are means ± SD of duplicate determinations from three independent experiments. # p < 0.001 versus the non-treated control group; * p < 0.05, ** p < 0.05, *** p < 0.01 versu PMA plus A23187 alone group.
Figure 3.
Inhibitory effect of luteolin on PMA plus A23187-induced TNF-α (A)- and IL-1β (B) production in HMC-1 cells. Cells (5 × 105) were pretreated with 1–20 μM luteolin for 30 min prior to stimulation with or without 25 nM PMA plus 1 μM A23187 and then incubated at 37 °C for 12 h. Cytokine levels were determined by enzyme-linked immunosorbent assay (ELISA). Values are means ± SD of duplicate determinations from three independent experiments. # p < 0.001 versus the non-treated control group; * p < 0.05, ** p < 0.05, *** p < 0.01 versu PMA plus A23187 alone group.
2.3. Inhibition of Histamine Release by Luteolin
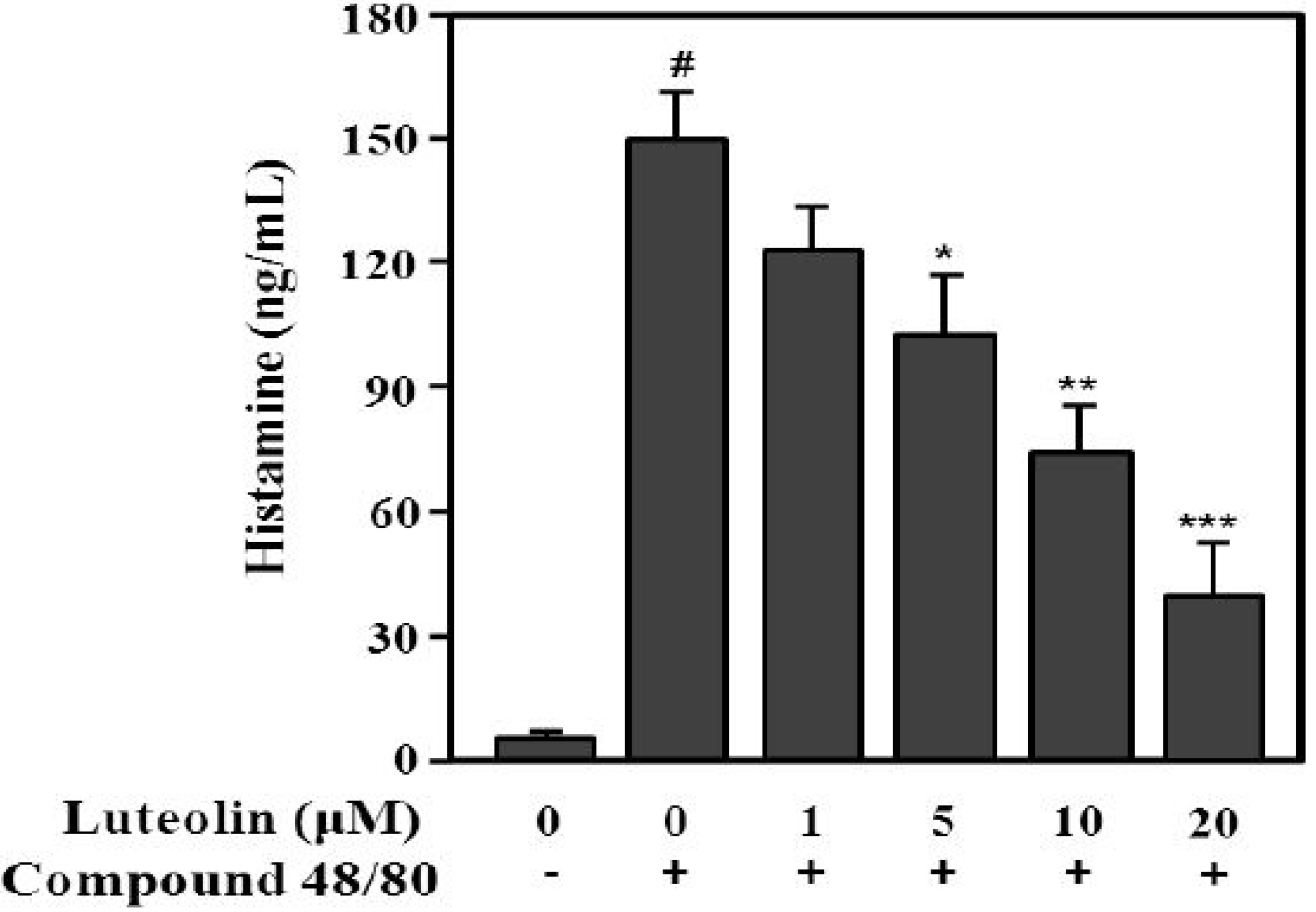
Compound 48/80 increases the permeability of the lipid bilayer by causing a perturbation in the membrane [26]. Additionally, compound 48/80 can activate the release of histamine from mast cells [27]. Next, to evaluate the inhibitory effect of luteolin on histamine release, RPMCs were treated with compound 48/80. As shown in Figure 4, compound 48/80 significantly enhanced histamine release (148.7 ± 14.9 ng/mL) compared with the control (6.7 ± 2.5 ng/mL; p < 0.001). Luteolin significantly inhibited the compound 48/80-induced histamine release at 5, 10, and 20 μM in a dose-dependent manner (p < 0.05, p < 0.01 and p < 0.001, respectively).
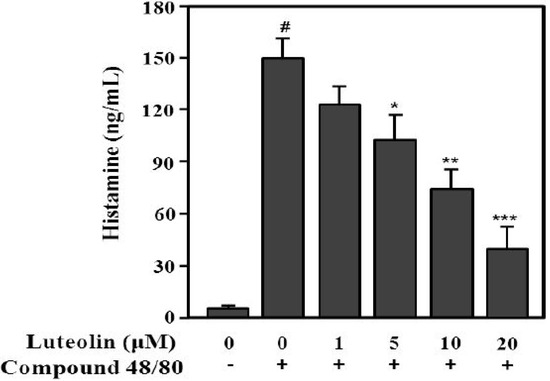
Figure 4.
Inhibitory effect of luteolin on histamine released from RPMCs activated with compound 48/80. RPMCs (5 × 105/mL) were pre-treated with or without luteolin at the indicated concentrations for 2 h, and then stimulated with or without compound 48/80 (5 μg/mL). Histamine levels in RPMC supernatants were determined by ELISA. Values are means ± SD of three independent experiments. # p < 0.001 versus the non-treated control group; * p < 0.05, ** p < 0.05, *** p < 0.01 versus compound 48/80 alone group.
Figure 4.
Inhibitory effect of luteolin on histamine released from RPMCs activated with compound 48/80. RPMCs (5 × 105/mL) were pre-treated with or without luteolin at the indicated concentrations for 2 h, and then stimulated with or without compound 48/80 (5 μg/mL). Histamine levels in RPMC supernatants were determined by ELISA. Values are means ± SD of three independent experiments. # p < 0.001 versus the non-treated control group; * p < 0.05, ** p < 0.05, *** p < 0.01 versus compound 48/80 alone group.
2.4. Effects of Luteolin on Scratching Behavior and Vascular Permeability
Compound 48/80 is a potent activator of connective tissue-type and/or skin mast cells [28]. Therefore, the scratching behavior caused by compound 48/80 may be affected via mediators released from mast cells, such as histamine and substance P [29,30]. Therefore, we determined whether luteolin could reduce the scratching behavior and skin vascular permeability induced by pruritogens (compound 48/80 or serotonin) in ICR mice. As shown in Figure 5, compound 48/80 markedly increased the scratching behavior (166.5 ± 16.4 times/60 min) and vascular permeability (100% ± 9.8%) compared with the control (9.8 ± 3.4 times/60 min, 14.5% ± 1.9%; p < 0.001).
Serotonin (5-HT) also markedly increased the scratching behavior (73.5 ± 10.3 times/min) and vascular permeability (100% ± 9.8%) compared with the control (p < 0.001), as shown in Figure 6. Luteolin significantly inhibited compound 48/80- or serotonin-induced scratching behavior at 5, 10 and 20 mg/kg in a dose-dependent manner (p < 0.05, p < 0.01 and p < 0.001, respectively).
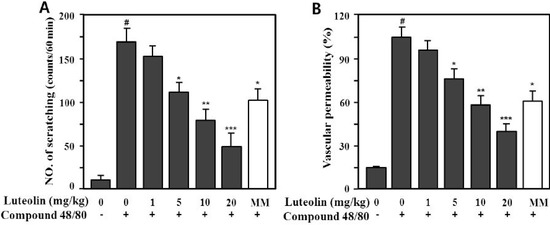
Figure 5.
Effect of luteolin on compound 48/80-induced scratching behavior (A) and vascular permeability (B) in mice. Scratching behavior was counted for 60 min after intradermal injection of compound 48/80 (50 μg/site). Luteolin (1–20 mg/kg) was orally administered 1 h before injection of compound 48/80. Values are means ± standard error (SE) (n = 8). # p < 0.001 versus the non-treated control group; * p < 0.05, ** p < 0.05, *** p < 0.01 versus compound 48/80 alone group.
Figure 5.
Effect of luteolin on compound 48/80-induced scratching behavior (A) and vascular permeability (B) in mice. Scratching behavior was counted for 60 min after intradermal injection of compound 48/80 (50 μg/site). Luteolin (1–20 mg/kg) was orally administered 1 h before injection of compound 48/80. Values are means ± standard error (SE) (n = 8). # p < 0.001 versus the non-treated control group; * p < 0.05, ** p < 0.05, *** p < 0.01 versus compound 48/80 alone group.
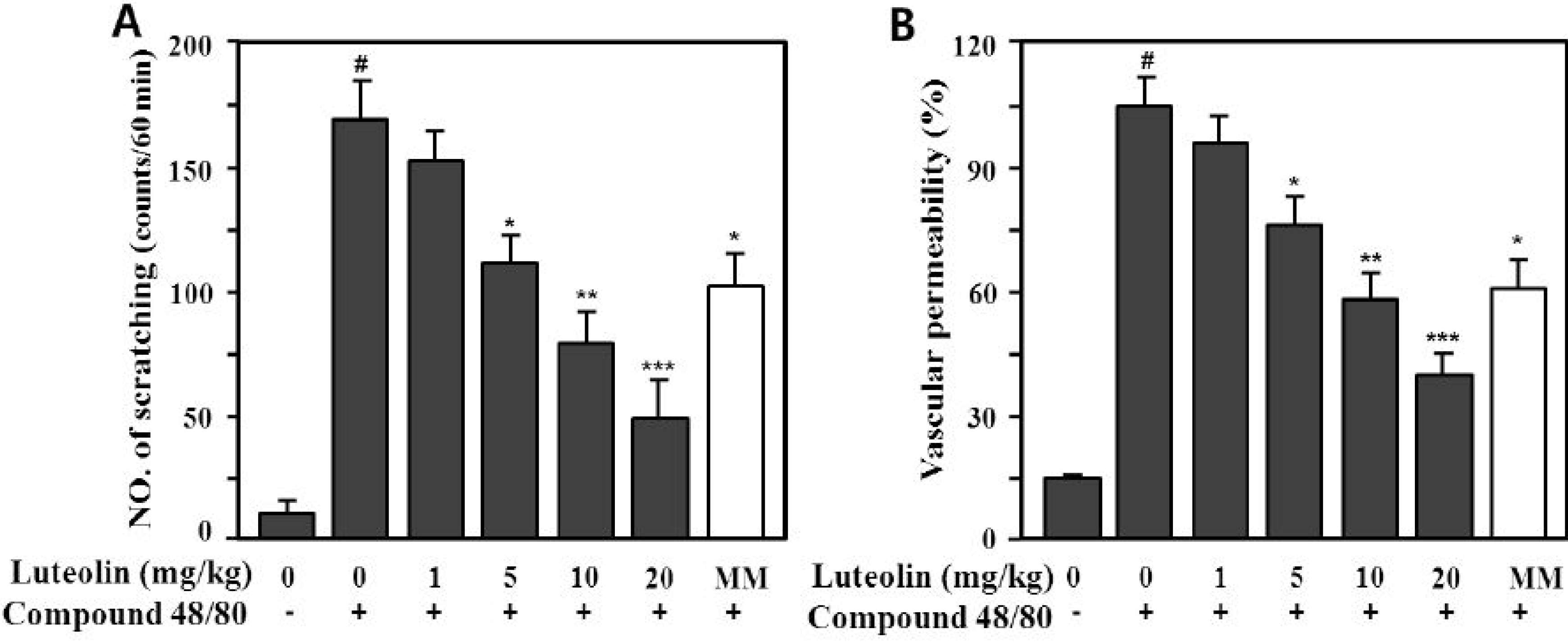
Figure 6.
Effect of luteolin on serotonin-induced scratching behavior (A) and vascular permeability (B) in ICR mice. Scratching behavior was counted for 60 min after intradermal injection of serotonin (100 μg/site). Luteolin (1–20 mg/kg) was administered orally 1 h before serotonin injection. Values are means ± SE (n = 8). # p < 0.001 versus the non-treated control group; * p < 0.05, ** p < 0.05, *** p < 0.01 versus serotonin alone group.
Figure 6.
Effect of luteolin on serotonin-induced scratching behavior (A) and vascular permeability (B) in ICR mice. Scratching behavior was counted for 60 min after intradermal injection of serotonin (100 μg/site). Luteolin (1–20 mg/kg) was administered orally 1 h before serotonin injection. Values are means ± SE (n = 8). # p < 0.001 versus the non-treated control group; * p < 0.05, ** p < 0.05, *** p < 0.01 versus serotonin alone group.
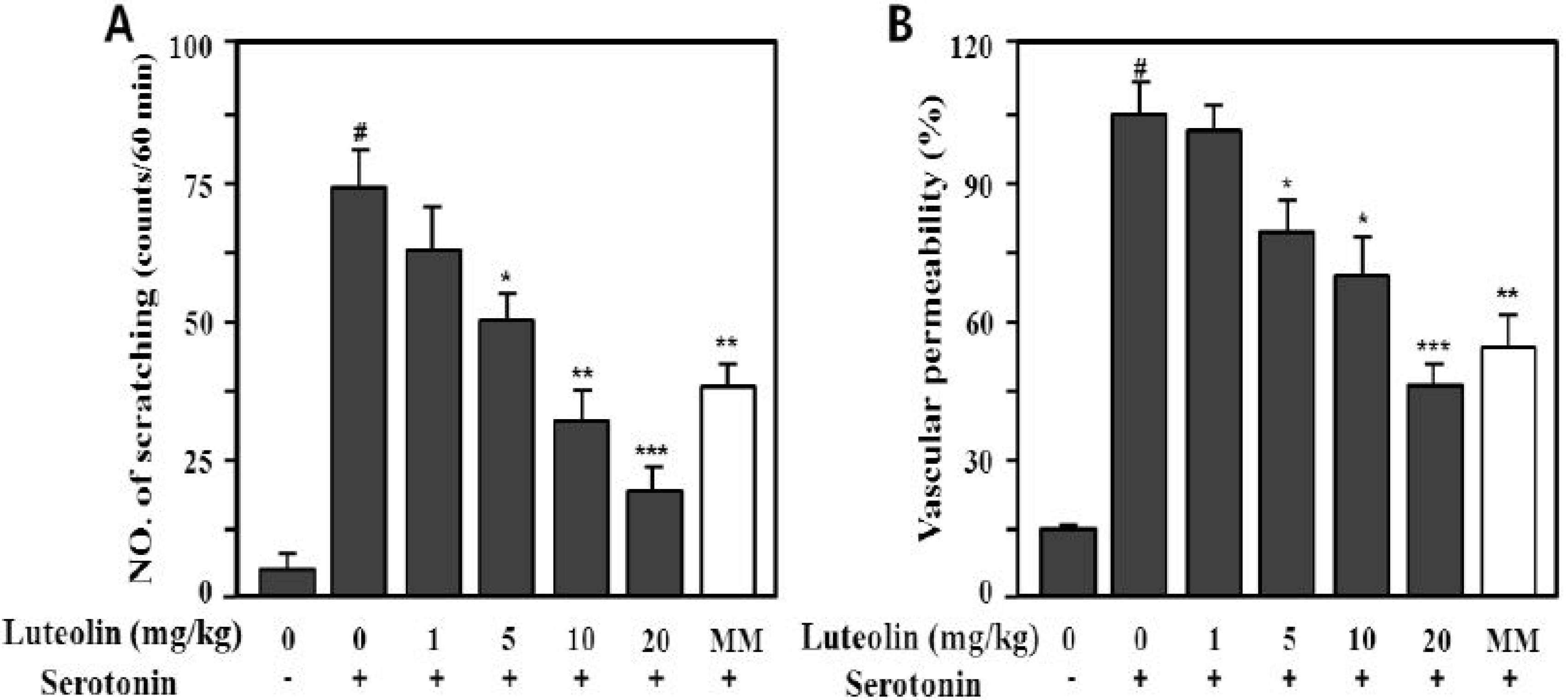
Methysergide interacts with serotonin (5-HT2B) receptors. Its therapeutic effect in migraine prophylaxis is associated with antagonism of the 5-HT2B receptor [31] and at a dose of 10 mg/kg has a significantly antagonistic effect on th 5-HT-induced mouse itching response. To investigate the antipruritic effect of luteolin, we used methysergide maleate (MM) as the reference drug. As shown in Figure 5A and Figure 6A, luteolin had a greater antipruritic effect than MM at the same concentration (10 mg/kg).
3. Experimental
3.1. Chemicals
Iscove’s modified Dulbecco’s medium (IMDM), glutamax-l and fetal bovine serum (FBS) were purchased from GIBCO-BRL, Invitrogen Co. (Grand Island, NY, USA). TNF-α and IL-1β ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA). Histamine ELISA kits were purchased from ALPCO Diagnostics (Salem, NH, USA). Compound 48/80, phorbol 12-myristate 13-acetate (PMA), penicillin/streptomycin (P/S), MM and other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Perilla Leaves and Luteolin Isolation
Perilla leaves were collected on June 20, 2012 from Aenong Farm (Jinan, Jeollabuk-do, Republic of Korea). The plant was identified and authenticated by Professor Hong-Jun Kim at the College of Oriental Medicine, Woosuk University. A voucher specimen (#ML-11-02) was deposited in the author’s laboratory (Professor Seon-Il Jang).
Dried perilla leaves (100 g) were extracted with MeOH (2 L) for 60 min at 25 °C. The MeOH extracts were partitioned with in sequence organic solvents of different polarities to yield n-hexane, EtOAc, n-BuOH and H2O fractions. The EtOAc fraction was subjected to silica gel chromatography with CH2Cl2–MeOH (lower layers, by volume, 30:1→1:1, 100% MeOH, gradient) as the solvent to yield the perilla luteolin (3',4',5,7-tetrahydroxylflavone). The structure of the compound (Figure 1) was determined by its physicochemical and spectral data (LC-MS, 1H- and 13C-NMR), which were in agreement with previously reported values [20].
3.3. Animals
Male ICR mice (6 weeks old) were purchased from Central Lab Inc. (Seoul, Korea), the Korean branch of Charles River Japan (Kanagawa, Japan). Male Sprague-Dawley rats (8 weeks old) were purchased from Oriental Bio Experimental Center (Gyeanggi-do, Republic of Korea). Animals were maintained in an environmentally controlled housing system and used for experiments after 1 week. The animals were given a standard laboratory diet and water ad libitum. All experiments were performed in accordance with Jeonju University Institutional Animal Care and Use Committee guidelines (No. 2012-002).
3.4. Cell Culture and Stimulation
HMC-1 cells were cultured in IMDM with glutamax-l supplemented with 10% FBS at 37 °C in a 5% CO2 atmosphere at 95% humidity. HMC-1 cells (5 × 105) were treated with 1–20 μM luteolin and then incubated at 37 °C for 8 h. The cell viability was over 95% in all experiments.
3.5. MTT Assay
Cell viability was determined by the MTT assay. Briefly, HMC-1 cells (5 × 105/well) were cultured in four-well plates for 8 h after treatment with the various luteolin concentrations. Twenty microliters of MTT solution (5 mg/mL) were added and the cells were incubated at 37 °C for a further 4 h. After washing out the supernatant, the insoluble formazan product was dissolved in DMSO. Next, the optical density at 540 nm in the 96-well culture plates was measured using a microplate reader.
3.6. Rat Peritoneal Mast Cell (RPMC) Isolation and Stimulation
The mice were anesthetized, and 10 mL of Tyrode buffer A (10 mM HEPES, 136 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2.75 mM MgCl2, 5.6 mM glucose, 11 mM NaHCO3, 0.56 mM NaH2PO4 and 0.1% bovine serum) containing 0.1% gelatin were injected into the peritoneal cavity. The cavity was carefully opened, and peritoneal cells were obtained and centrifuged at 150 ×g for 10 min at room temperature before resuspension in Tyrode buffer A. Mast cells were isolated from the peritoneal cells according to a method described previously [22] and then assessed by toluidine blue staining.
3.7. Histamine Assay
RPMCs were resuspended in Tyrode buffer A for treatment with compound 48/80. RPMC suspensions (5 × 105/mL) were preincubated with 1–20 μM luteolin and then stimulated with compound 48/80 (5 μg/mL). Each activated RPMC was centrifuged at 150 ×g for 10 min at 4 °C and the supernatant obtained. Each supernatant was then assayed for histamine content by ELISA according to the manufacturer’s specifications. Absorption of the avidin-horseradish peroxidase color reaction was measured at 450 nm.
3.8. Cytokine ELISA
Secreted cytokine levels in culture supernatants of HMC-1 cells were measured using a sandwich ELISA according to the manufacturer’s protocol (for IL-1β and TNF-α assay, R&D Systems). Absorption of the avidin-horseradish peroxidase color reaction was measured at 450 nm.
3.9. Scratching Behavior
Before the experiment, ICR mice were put into acrylic cages (22 cm × 22 cm × 24 cm) for about 10 min for acclimation. Scratching behavior was evaluated as described previously [11,32]. Briefly, luteolin (1–20 mg/kg, body weight) or MM (10 mg/kg) dissolved in PBS was administered orally to the mice. One hour later, compound 48/80 (50 μg/site) or serotonin (100 μg/site) was injected intradermally into the rostral part of the shaved back of the mouse. Normal control mice were injected with the same volume of PBS. Immediately after injection, the mice (one mouse/cage) were placed into an observation chamber and scratching behavior was recorded using a micro camera (ONCCTV, Seoul, Korea). Scratching actions with the hind paw were enumerated for 60 min and recorded for analysis.
3.10. Skin Vascular Permeability
The increase in skin vascular permeability caused by scratching agents was assessed as reported previously [33]. After intradermal injection of compound 48/80 (50 μg/site) or serotonin (100 μg/site) into the rostral part of the mouse back, 2% Evans blue solution was injected intravenously into each animal. The animals were sacrificed 60 min later and the scratching agent-injected site (1 × 1 cm) was immediately excised. The skin specimen was dissolved with 1 mL of 1 M KOH solution by overnight incubation and 4 mL of 0.2 M phosphoric acid-acetone mixture were added. After vigorous shaking, the precipitates were filtered and dye was quantified colorimetrically at 620 nm.
3.11. Statistical Analysis
Differences among the groups were evaluated by one-way ANOVA, and all values were expressed as means ± standard deviation (SD). Differences among groups were considered significant at p < 0.05.
4. Conclusions
In conclusion, luteolin from perilla leaves inhibited the production of TNF-α and IL-1β in PMA plus A23187-activated HMC-1cells. Luteolin also suppressed histamine release by compound 48/80-stimulated RPMCs. Furthermore, luteolin reduced the scratching behavior and vascular permeability induced by compound 48/80 or serotonin, thus exhibiting significant anti-inflammatory and antipruritic effects on inflammation and the itching response. Moreover, these results suggest that luteolin has potential as a therapeutic agent for allergic diseases.
Acknowledgments
This work was supported by the Small and Medium Business administration (No. C0101031), the Jeollabuk-do (2013HaC11)) and the Ministry of Trade, Industry & Energy (No. R0002271) of Republic of Korea in 2013.
Author Contributions
S.I.J. and I.H.J participated in research design. H.J.K., H.S.K., I.H.J. and H.S.L. performed cell culture and scratching behavior experiments. The luteolin structure was confirmed by S.J.K and S.I.J. The manuscript was written by S.I.J.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hougee, S.; Sanders, A.; Faber, J.; Graus, Y.M.; van den Berg, W.B.; Garssen, J.; Smit, H.F.; Hoijer, M.A. Decreased pro-inflammatory cytokine production by LPS-stimulated PBMC upon in vitro incubation with the flavonoids apigenin, luteolin or chrysin, due to selective elimination of monocytes/macrophages. Biochem. Pharmacol. 2005, 69, 241–248. [Google Scholar]
- Suárez, A.L.; Feramisco, J.D.; Koo, J.; Steinhoff, M. Psychoneuroimmunology of psychological stress and atopic dermatitis: Pathophysiologic and therapeutic updates. Acta Derm. Venereol. 2012, 92, 7–15. [Google Scholar]
- Gurish, M.F.; Austen, K.F. The diverse roles of mast cells. J. Exp. Med. 2001, 94, F1–F5. [Google Scholar]
- Kim, E.K.; Kim, E.Y.; Moon, P.D.; Um, J.Y.; Kim, H.M.; Lee, H.S.; Sohn, Y.; Park, S.K.; Jung, H.S.; Sohn, N.W. Lithospermi radix extract inhibits histamine release and production of inflammatory cytokine in mast cells. Biosci. Biotech. Biochem. 2007, 71, 2886–1892. [Google Scholar]
- Hosoda, M.; Yamaya, M.; Suzuki, T.; Yamada, N.; Kamanakam, M.; Sekizawa, K.; Butterfield, J.H.; Watanabe, T.; Nishimura, H.; Sasaki, H. Effects of rhinovirus infection on histamine and cytokine production by cell lines from human mast cells and basophils. J. Immunol. 2002, 169, 1482–1491. [Google Scholar]
- Rasheed, Z.; Akhtar, H.; Khan, A.; Khan, K.A.; Haqqi, T.M. Butrin, isobutrin, and butein from medicinal plant Butea monosperma selectively inhibit nuclear factor-κB in activated human mast cells: Suppression of tumor necrosis factor-α, interleukin (IL)-6, and IL-8. J. Pharmacol. Exp. Ther. 2010, 333, 354–363. [Google Scholar]
- Mekori, Y.A.; Metcalfe, D. Mast cells in innate immunity. Immunol. Rev. 2000, 173, 131–140. [Google Scholar]
- Ikoma, A.; Steinhoff, M.; Ständer, S.; Yosipovitch, G.; Schmelz, M. The neurobiology of itch. Nat. Rev. Neurosci. 2006, 7, 535–547. [Google Scholar]
- Steinhoff, M.; Bienenstock, J.; Schmelz, M.; Maurer, M.; Wei, E.; Bíró, T. Neurophysiological, neurological, and neuroendocrine basis of pruritus. J. Invest. Dermatol. 2006, 126, 1705–1718. [Google Scholar]
- Hong, J.; Buddenkotte, J.; Berger, T.G.; Steinhoff, M. Management of itch in atopic dermatitis. Semin. Cutan. Med. Surg. 2011, 30, 71–86. [Google Scholar]
- Kobayashi, Y.; Takahashi, R.; Ogino, F. Antipruritic effect of the single oral administration of German chamomile flower extract and its combined effect with antiallergic agents in ddY mice. J. Ethnopharmacol. 2005, 101, 308–312. [Google Scholar]
- Liu, J.; Wan, Y.; Zhao, Z.; Chen, H. Determination of the content of rosmarinic acid by HPLC and analytical comparison of volatile constituents by GC-MS in different parts of Perilla frutescens (L.). Britt. Chem. Cent. J. 2013, 7, 1–11. [Google Scholar]
- Kim, D.H.; Kim, Y.C.; Choi, U.K. Optimization of antibacterial activity of Perilla frutescens var. acuta leaf against Staphylococcus aureus using evolutionary operation factorial design technique. Int. J. Mol. Sci. 2011, 12, 2395–2407. [Google Scholar]
- Makino, T.; Furuta, Y.; Wakushima, H.; Fujii, H.; Saito, K.; KanoY., Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytother. Res. 2003, 17, 240–243. [Google Scholar]
- Seo, W.H.; Baek, H.H. Characteristic aroma-active compounds of Korean perilla (Perillafrutescens Britton) leaf. J. Agric. Food Chem. 2009, 57, 11537–11542. [Google Scholar]
- Ragazinskiene, O.; Gailys, V.; Jankauskiene, K.; Simoniene, G.; Jurkstiene, V. Common perilla (Perilla frutescens (L.) Britton.) as a perspective immunomodulator. Medicina (Kaunas) 2004, 40, 220–224. [Google Scholar]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol. Pharmaceut. Bull. 2002, 25, 1197–1202. [Google Scholar]
- Haghi, G.; Hatami, A. Simultaneous quantification of flavonoids and phenolic acids in plant materials by a newly developed isocratic high-performance liquid chromatography approach. J. Agric. Food Chem. 2010, 58, 10812–10816. [Google Scholar]
- Harris, G.K.; Qian, Y.; Leonard, S.S.; Sbarra, D.C.; Shi, X. Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-E2 formation in RAW 264.7 cells. J. Nutri. 2006, 136, 1517–1521. [Google Scholar]
- Choi, C.W.; Jung, H.A.; Kang, S.S.; Choi, J.S. Antioxidant constituents and a new triterpenoid glycoside from Flos Lonicera. Arch. Pharm. Res. 2007, 30, 1–7. [Google Scholar]
- Chan, B.C.; Hon, K.L.; Leung, P.C.; Sam, S.W.; Fung, K.P.; Lee, M.Y.; Lau, H.Y. Traditional Chinese medicine for atopic eczema: Penta herbs formula suppresses inflammatory mediators release from mast cells. J. Ethnopharmacol. 2008, 120, 85–91. [Google Scholar]
- Chan, C.L.; Jones, R.L.; Lau, H.Y. Characterization of prostanoid receptors mediating inhibition of histamine release from anti-IgE-activated rat peritoneal mast cells. Br. J. Pharmacol. 2000, 129, 589–597. [Google Scholar]
- Kang, O.H.; Choi, J.G.; Lee, J.H.; Kwon, D.Y. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-kappaB and APKs activation pathways in HMC-1 cells. Molecules 2010, 15, 385–398. [Google Scholar]
- Stassen, M.; Müller, C.; Arnold, M.; Hültner, L.; Klein-Hessling, S.; Neudörfl, C.; Reineke, T.; Serfling, E.; Schmitt, E. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-kappa B is decisively involved in the expression of IL-9. J. Immunol. 2000, 166, 4391–4398. [Google Scholar]
- Guma, M.; Kashiwakura, J.I.; Crain, B.; Kawakami, Y.; Beutler, B.; Firestein, G.S.; Kawakami, T.; Karin, M.; Corr, M. JNK1 controls mast cell degranulation and IL-1β production in inflammatory arthritis. Proc. Natl. Acad. Sci. USA 2010, 107, 22122–22127. [Google Scholar]
- Kim, C.D.; Lee, W.K.; No, K.O.; Park, S.K.; Lee, M.H.; Lim, S.R.; Roh, S.S. Anti-allergic action of buckwheat (Fagopyrum esculentum Moench) grain extract. Int. Immunopharmacol. 2003, 3, 129–136. [Google Scholar]
- Choi, Y.H.; Chai, O.H.; Han, E.H.; Choi, S.Y.; Kim, H.T.; Song, C.H. Lipoic acid suppresses compound 48/80-induced anaphylaxis-like reaction. Anat. Cell Biol. 2010, 43, 317–324. [Google Scholar]
- Rukwied, R.; Lischetzki, G.; McGlone, F.; Heyer, G.; Schmilz, M. Mast cell mediators other than histamine induce pruritus in atopic dermatitis patterns: A dermal microdialysis study. Br. J. Pharmacol. 2000, 142, 1114–1120. [Google Scholar]
- Inagaki, N.; Igeta, K.; Kim, J.F.; Nagao, M.; Shiraishi, N.; Nakamura, N.H.; Nagai, H. Involvement of unique mechanisms in the induction of scratching behavior in BALB/c mice by compound 48/80. Eur. J. Pharmacol. 2002, 448, 175–183. [Google Scholar]
- Ohmura, T.; Hayashi, T.; Satoh, Y.; Konomi, A.; Jung, B.; Sato, H. Involvement of substance P in scratching behavior in an atopic dermatitis model. Eur. J. Pharmacol. 2004, 491, 191–194. [Google Scholar]
- Stephane, D.; Emmanuel, V.; Vincent, S.; Jacques, C.; Denis, H.; Jean-Marie, L.; Luc, M. Serotonin 5-HT2B receptors are required for 3,4-Methylenedioxymethamphetamine-induced hyperlocomotion and 5-HT release in vivo and in vitro. J. Neurosci. 2008, 28, 2933–2940. [Google Scholar]
- Takubo, M.; Ueda, Y.; Yatsuzuka, R.; Jiang, S.; Fujii, Y.; Kamei, C. Characteristics of scratching behavior induced by some chemical mediators in hairless mice. J. Pharmacol. Sci. 2006, 100, 285–288. [Google Scholar]
- Sugimoto, Y.; Umakoshi, K.; Nojiri, N.; Kamei, C. Effect of histamine H1 receptor antagonists on compound-induced scratching behavior in mice. Eur. J. Pharmacol. 1998, 351, 1–5. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
