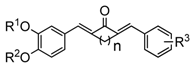
Synthesis and Evaluation of a Series of Novel Asymmetrical Curcumin Analogs for the Treatment of Inflammation
Abstract
:1. Introduction
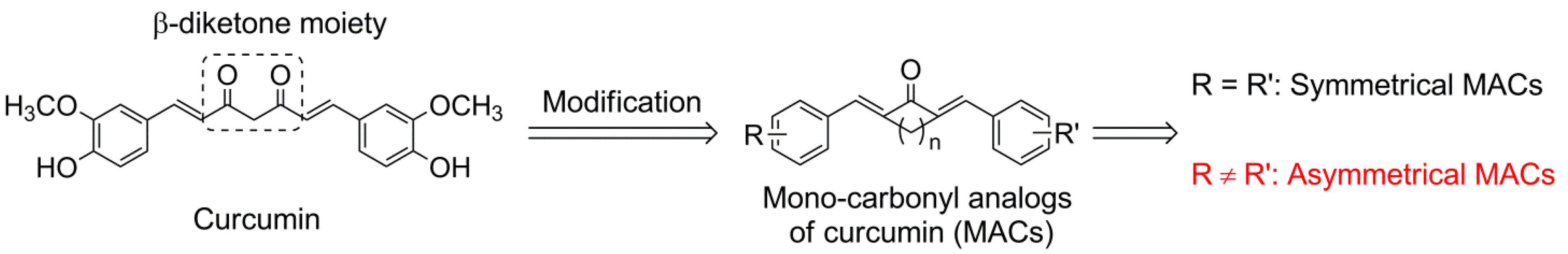
2. Results and Discussion
2.1. Chemistry

| Comp. | R1 | R2 | R3 | n | Comp. | R1 | R2 | R3 | n |
|---|---|---|---|---|---|---|---|---|---|
| 3a1 | H | H | 3,4-OMe | 3 | 3c6 | Me | propionyl | 3-OMe, 4-O-propionyl | 3 |
| 3a2 | H | H | 3,4,5-OMe | 2 | 3d1 | Me | isobutyryl | 2-F | 2 |
| 3a3 | H | H | 3,4,5-OMe | 3 | 3d2 | Me | isobutyryl | 4-F | 2 |
| 3b1 | Me | H | 2-F | 2 | 3d3 | Me | isobutyryl | 2-OMe | 2 |
| 3b2 | Me | H | 4-F | 2 | 3d4 | Me | isobutyryl | 4-F | 2 |
| 3b3 | Me | H | 2-Cl | 2 | 3d5 | Me | isobutyryl | 4-OEt | 2 |
| 3b4 | Me | H | 4- tert-butyl | 2 | 3d6 | Me | isobutyryl | 3,4-OMe | 2 |
| 3b5 | Me | H | 2-OMe | 2 | 3d7 | Me | isobutyryl | 2,5-OMe | 2 |
| 3b6 | Me | H | 3-OH, 4-OMe | 2 | 3d8 | Me | isobutyryl | 3,4,5-OMe | 2 |
| 3b7 | Me | H | 4- di-ethylamino | 2 | 3d9 | Me | isobutyryl | 2-O-isobutyryl | 2 |
| 3b8 | Me | H | 4- di-butylamino | 2 | 3d10 | Me | isobutyryl | 4-O-isobutyryl | 2 |
| 3b9 | Me | H | 4-piperidine | 2 | 3d11 | Me | isobutyryl | 3-O-isobutyryl, 4-OMe | 2 |
| 3b10 | Me | H | 4-morpholine | 2 | 3d12 | Me | isobutyryl | 3,4-O-isobutyryl | 2 |
| 3c1 | Me | H | 2-F | 2 | 3d13 | Me | isobutyryl | 4- di-ethylamino | 2 |
| 3c2 | Me | propionyl | 4- di-ethylamino | 2 | 3d14 | Me | isobutyryl | 4-morpholine | 2 |
| 3c3 | Me | propionyl | 4-piperidine | 2 | 3d15 | Me | isobutyryl | 3-OMe,4-O-isobutyryl | 0 |
| 3c4 | Me | propionyl | 4-morpholine | 2 | 3d16 | Me | isobutyryl | 3-OMe,4-O-isobutyryl | 3 |
| 3c5 | Me | propionyl | 3-OMe, 4-O-propionyl | 2 |
2.2. Biological Evaluation
2.2.1. Anti-Inflammatory Activity

2.2.2. Quantitative Structure-Activity Relationships (QSARs)

2.2.3. Six Active Compounds Inhibit TNF-α and IL-6 Release in a Dose-Dependent Manner

2.2.4. The Active Compounds Showed Much Higher Chemical Stability than Curcumin

2.2.5. Cell Toxicity Assay

2.2.6. 3b8 and 3b9 Attenuated the LPS-Induced Septic Death in Mice

3. Experimental
3.1. General Information
3.2. Chemical Synthesis
3.2.1. Synthesis of 2a and 2b
3.2.2. Synthesis of 3a1–3a3, and 3b1–3b10
3.2.3. Synthesis of 3c1–3c4 and 3d1–3d14
3.2.4. Synthesis of 3c5, 3c6, 3d15, and 3d16
3.3. Animals
3.4. Cell Treatment and ELISA
3.5. Descriptor Calculation and Selection
3.6. Multiple Linear Regression (MLR) Analysis
3.7. Validation of the Models
3.8. In Vivo Study
3.9. The Stability Analysis of Curcumin and Its Analogs by HPLC
3.10. Cell Viability Assay
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- O’Sullivan-Coyne, G.; O’Sullivan, G.C.; O’Donovan, T.R.; Piwocka, K.; McKenna, S.L. Curcumin induces apoptosis-independent death in oesophageal cancer cells. Br. J. Cancer 2009, 101, 1585–1595. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Thakare, V.N.; Osama, M.M.; Naik, S.R. Therapeutic potential of curcumin in experimentally induced allergic rhinitis in guinea pigs. Int. Immunopharmacol. 2013, 17, 18–25. [Google Scholar] [CrossRef]
- Huang, G.; Xu, Z.; Huang, Y.; Duan, X.; Gong, W.; Zhang, Y.; Fan, J.; He, F. Curcumin protects against collagen-induced arthritis via suppression of BAFF production. J. Clin. Immunol. 2013, 33, 550–557. [Google Scholar] [CrossRef]
- Kusuma, A.; Colpitts, C.C.; Schang, L.M.; Rachmawati, H.; Frentzen, A.; Pfaender, S.; Behrendt, P.; Brown, R.J.P.; Bankwitz, D.; Steinmann, J.; et al. Turmeric Curcumin Inhibits Entry of All Hepatitis C Virus Genotypes into Human Liver Cells. J. Hepatol. 2013, 58, S473–S473. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in micse. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Lin, J.K.; Pan, M.H.; Lin-Shiau, S.Y. Recent studies on the biofunctions and biotransformations of curcumin. BioFactors 2000, 13, 153–158. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Rosemond, M.J.; St. John-Williams, L.; Yamaguchi, T.; Fujishita, T.; Walsh, J.S. Enzymology of a carbonyl reduction clearance pathway for the HIV integrase inhibitor, S-1360: Role of human liver cytosolic aldo-keto reductases. Chem.-Biol. Interact. 2004, 147, 129–139. [Google Scholar] [CrossRef]
- Grogan, G. Emergent mechanistic diversity of enzyme-catalysed beta-diketone cleavage. Biochem. J. 2005, 388, 721–730. [Google Scholar] [CrossRef]
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg. Med. Chem. 2009, 17, 2623–2631. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, J.; Wang, Y.; Liang, D.; Yang, X.; Li, X.; Wu, J.; Wu, X.; Yang, S.; Li, X. Synthesis of mono-carbonyl analogues of curcumin and their effects on inhibition of cytokine release in LPS-stimulated RAW 264.7 macrophages. Bioorg. Med. Chem. 2010, 18, 2388–2393. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.; Liang, G. Promising curcumin-based drug design: Mono-carbonyl analogues of curcumin (MACs). Curr. Pharm. Des. 2013, 19, 2114–2135. [Google Scholar]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef]
- Hudson, B.I.; Bucciarelli, L.G.; Wendt, T.; Sakaguchi, T.; Lalla, E.; Qu, W.; Lu, Y.; Lee, L.; Stern, D.M.; Naka, Y. Blockade of receptor for advanced glycation endproducts: A new target for therapeutic intervention in diabetic complications and inflammatory disorders. Arch. Biochem. Biophys. 2003, 419, 80–88. [Google Scholar] [CrossRef]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar]
- Jackson, J.; Higo, T.; Hunter, W.; Burt, H. The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm. Res. 2006, 55, 168–175. [Google Scholar] [CrossRef]
- Bengmark, S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: A shield against acute and chronic diseases. J. Parenter. Enter. Nutr. 2006, 30, 45–51. [Google Scholar] [CrossRef]
- Stork, G.; Dowd, S.R. A New Method for the Alkylation of Ketones and Aldehydes: The C-Alkylation of the Magnesium Salts of N-Substituted Imines. J. Am. Chem. Soc. 1963, 85, 2178–2180. [Google Scholar] [CrossRef]
- Kim, S.J. Curcumin suppresses the production of interleukin-6 in Prevotella intermedia lipopolysaccharide-activated RAW 264.7 cells. J. Periodontal Implant Sci. 2011, 41, 157–163. [Google Scholar] [CrossRef]
- Xue, Y.; Li, H.; Ung, C.Y.; Yap, C.W.; Chen, Y.Z. Classification of a diverse set of Tetrahymena pyriformis toxicity chemical compounds from molecular descriptors by statistical learning methods. Chem. Res. Toxicol. 2006, 19, 1030–1039. [Google Scholar] [CrossRef]
- Lukita-Atmadja, W.; Ito, Y.; Baker, G.L.; McCuskey, R.S. Effect of curcuminoids as anti-inflammatory agents on the hepatic microvascular response to endotoxin. Shock 2002, 17, 399–403. [Google Scholar] [CrossRef]
- Maggiora, G.M. On outliers and activity cliffs—Why QSAR often disappoints. J. Chem. Inf. Model. 2006, 46, 1535–1535. [Google Scholar] [CrossRef]
- Stewart, J.J. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef]
- Li, Z.R.; Han, L.Y.; Xue, Y.; Yap, C.W.; Li, H.; Jiang, L.; Chen, Y.Z. MODEL-molecular descriptor lab: A web-based server for computing structural and physicochemical features of compounds. Biotechnol. Bioeng. 2007, 97, 389–396. [Google Scholar] [CrossRef]
- Frick, W.E.; Ge, Z.; Zepp, R.G. Nowcasting and forecasting concentrations of biological contaminants at beaches: A feasibility and case study. Environ. Sci. Technol. 2008, 42, 4818–4824. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Y.; Zhao, L.; Wu, J.; Jiang, X.; Dong, L.; Xu, F.; Zou, P.; Dai, Y.; Shan, X.; Yang, S.; et al. Synthesis and Evaluation of a Series of Novel Asymmetrical Curcumin Analogs for the Treatment of Inflammation. Molecules 2014, 19, 7287-7307. https://doi.org/10.3390/molecules19067287
Zhang Y, Zhao L, Wu J, Jiang X, Dong L, Xu F, Zou P, Dai Y, Shan X, Yang S, et al. Synthesis and Evaluation of a Series of Novel Asymmetrical Curcumin Analogs for the Treatment of Inflammation. Molecules. 2014; 19(6):7287-7307. https://doi.org/10.3390/molecules19067287
Chicago/Turabian StyleZhang, Yali, Leping Zhao, Jianzhang Wu, Xin Jiang, Lili Dong, Fengli Xu, Peng Zou, Yuanrong Dai, Xiaoou Shan, Shulin Yang, and et al. 2014. "Synthesis and Evaluation of a Series of Novel Asymmetrical Curcumin Analogs for the Treatment of Inflammation" Molecules 19, no. 6: 7287-7307. https://doi.org/10.3390/molecules19067287
APA StyleZhang, Y., Zhao, L., Wu, J., Jiang, X., Dong, L., Xu, F., Zou, P., Dai, Y., Shan, X., Yang, S., & Liang, G. (2014). Synthesis and Evaluation of a Series of Novel Asymmetrical Curcumin Analogs for the Treatment of Inflammation. Molecules, 19(6), 7287-7307. https://doi.org/10.3390/molecules19067287