The Fruits of Wampee Inhibit H2O2-Induced Apoptosis in PC12 Cells via the NF-?B Pathway and Regulation of Cellular Redox Status
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of the New Compound 3

2.2. Analysis of the Constituents of CLS and Quantitation of Lutin and Quercetin-7-O-β-l-gluco- pyranoside in CLS
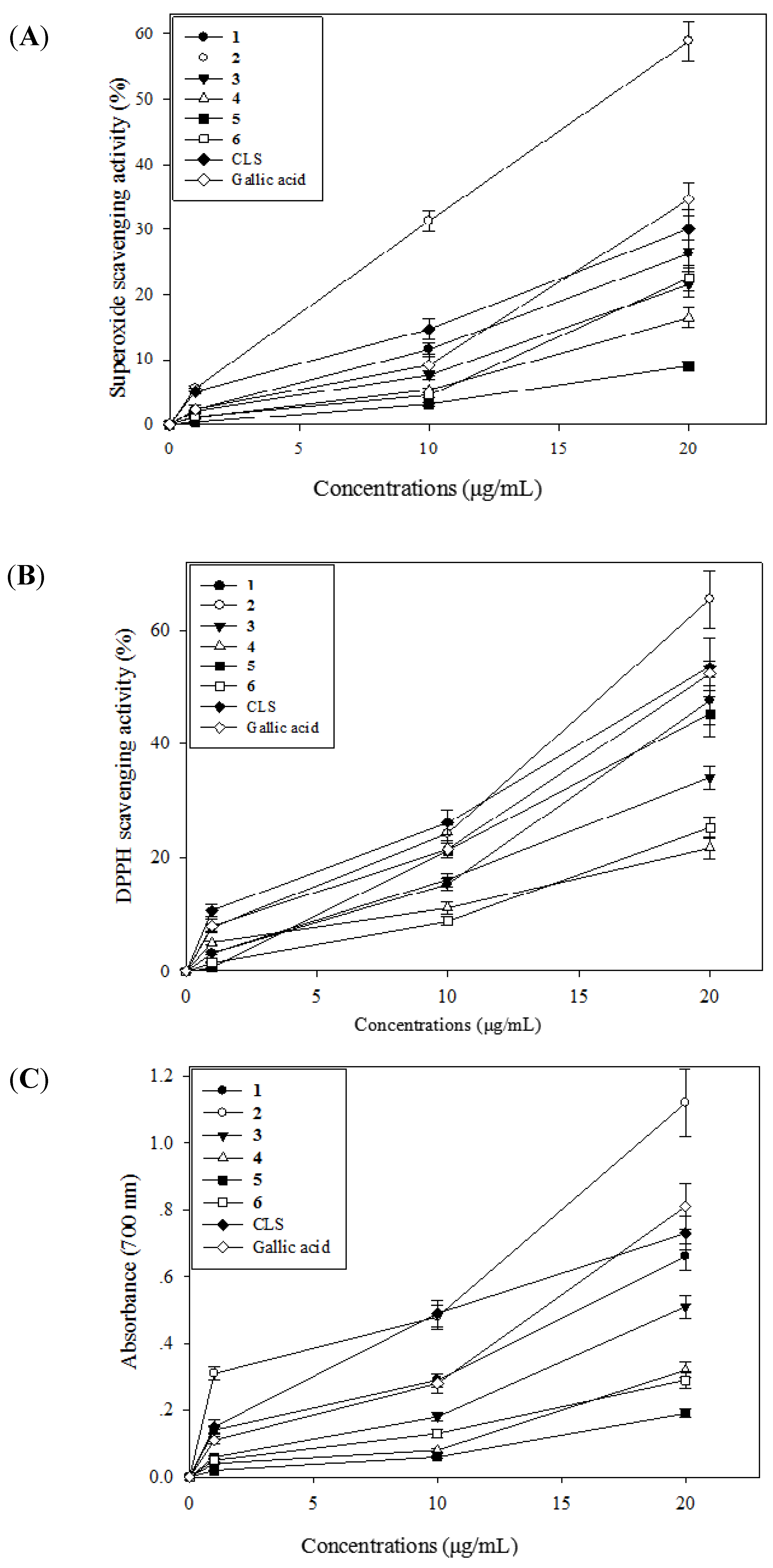
2.3. Antioxidative Activity of CLS and Its Isolates 1–6
2.4. Effects of CLS and its Isolates 1–6 on Cell Viability in PC12 Cells

2.5. Effects of CLS on DNA Condensation, Intracellular ROS Production and MMP Loss in PC12 Cells

2.6. Effects of CLS on the Pathway of NF-κB


2.7. Effects of CLS on Caspase-3 Expression
3. Experimental
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Luteolin-4'-O-β-d-glucopyranoside (3)
3.5. Analysis of Total Phenolic Content and Total Flavonoid Content
3.6. HPLC Analysis and Quantitation of Lutin and Quercetin-7-O-β-l-glucopranoside in CLS
3.7. Determination of in Vitro Antioxidant Activity
3.8. Cell Culture
3.9. Cell Viability Assay
3.10. DAPI Staining Analysis
3.11. Measurement of Intracellular ROS Accumulation
3.12. Measurement of Intracellular Mitochondrial Membrane Potential (MMP)
3.13. Immunostaining Assay
3.14. Western Blot Analysis
3.15. Statistical Analysis
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Jin, M.M.; Zhang, L.; Yu, H.X.; Meng, J.; Sun, Z.; Lu, R.R. Protective effect of whey protein hydrolysates on H2O2-induced PC12 cells oxidative stress via a mitochondria-mediated pathway. Food Chem. 2013, 141, 847–852. [Google Scholar]
- Benzi, G.; Moretti, A. Age and peroxidative stress-related modifications of the cerebral enzymatic activities linked to mitochondria and glutathione system. Free. Rad. Biol. Med. 1995, 19, 77–101. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of aging. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Liu, G.T.; Li, W.X.; Chen, Y.Y.; Wei, H.L. Hepatoprotective action of nine constituents isolated from the leaves of Clausena. lansium in mice. Drug. Dev. Res. 1996, 39, 174–178. [Google Scholar] [CrossRef]
- Ma, N.; Wu, K.; Huang, L. An elegant synthesis of Zetaclausenamide. Eur. J. Med. Chem. 2008, 43, 893–896. [Google Scholar] [CrossRef]
- Ng, T.B.; Lam, S.K.; Fong, W.P. A homodimeric sporamin-type trypsin inhibitor with antiproliferative, HIV reverse transcriptaseinhibitory and antifungal activities from wampee (Clausena lansium) seeds. Biol. Chem. 2003, 384, 289–293. [Google Scholar]
- Fan, G.J.; Han, B.H.; Kang, Y.H.; Park, M.K. Evaluation of inhibitory potentials of Chinese medicinal plants on platelet-activating factor (PAF) receptor binding. Nat. Prod. Sci. 2001, 7, 33–37. [Google Scholar]
- Prasad, K.N.; Hao, J.; Yi, C.; Zhang, D.D.; Qiu, S.X.; Jiang, Y.M.; Zhang, M.; Chen, F. Antioxidant and anticancer activity of Wampee (Clausena. lansium (Lour.) Skeels) Peel. J. Biomed. Biotech. 2009, 612805, 1–6. [Google Scholar]
- Adebajo, A.C.; Iwalewa, E.O.; Obuotor, E.M.; Ibikunle, G.F.; Omisore, N.O.; Adewunmi, C.O.; Schmidt, T.J.; Verspohl, E.J. Pharmacological properties of the extract and some isolated compounds of Clausena. lansium stem bark: Antitrichomonal, antidiabetic, antiinflammatory, hepatoprotective and antioxidant effects. J. Ethnopharm. 2008, 122, 10–19. [Google Scholar]
- Satoh, I.; Sakai, N.; Enokido, Y.; Uchiyama, Y.; Hatanaka, H. Free radical independent protection by nerve growth factor and Bcl-2 of PC12 cells from hydrogen peroxide triggered-apoptosis. J. Biochem. 1996, 120, 540–546. [Google Scholar] [CrossRef]
- Li, Y.; Guo, S.X.; Wang, C.L.; Xiao, P.G. Studies on flavonoids of Saussurea. involucrate. Chin. Pharm. J. 2007, 42, 575–577. [Google Scholar]
- Wu, T.; Abdulla, R.; Yang, Y.; Aisa, H.A. Flavonoids from Gossypium. hirsutum flowers. Chem. Nat. Comp. 2008, 44, 370–371. [Google Scholar] [CrossRef]
- Yang, M.H.; Chen, Y.Y.; Huang, L. Three novel cyclic amides from Clausena lansium. Phytochemistry 1988, 27, 445–450. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Liu, H.K.; Lo, J.M.; Chien, S.C.; Chan, Y.F.; Lee, T.H.; Su, J.K.; Kuo, Y.H. Cytotoxic constituents of the leaves of Calocedrus formosana. J. Chin. Chem. Soc. 2003, 50, 161–166. [Google Scholar]
- Zoecklin, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Chapman and Hall: New York, NY, USA, 1995. [Google Scholar]
- Moreno, M.I.N.; Isla, M.I.; Sampietro, A.R. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J. Ethnopharm. 2000, 71, 109–114. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar]
- Zeng, X.B.; Qiu, Q.; Jiang, C.G.; Jing, Y.T.; Qiu, G.F.; He, X.J. Antioxidant flavanes from Livistona chinensis. Fitoterapia 2011, 82, 609–614. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survivals: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- He, X.J.; Liu, R.H. Phytochemicals of apple peels: Isolation, structure elucidation, and their antiproliferative and antioxidant activities. J. Agric. Food. Chem. 2007, 55, 4366–4370. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhou, F.; Wu, X.L.; Zhang, X.X.; Chen, Y.C.; Zha, B.S.; Niu, F.; Lu, M.; Hao, G.; Sun, Y.; et al. Cellular pharmacokinetic mechanisms of adriamycin resistance and its modulation by 20(S)-ginsenoside Rh2 in MCF-7/Adr cells. Br. J. Pharm. 2012, 165, 120–134. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Calissano, P.; Matrone, C.; Amadoro, G. Apoptosis and in vitro Alzheimer disease neuronal models. Commun. Integr. Biol. 2009, 2, 163–169. [Google Scholar]
- Taupin, P. A dual activity of ROS and oxidative stress on adult neurogenesis and Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 16–21. [Google Scholar] [CrossRef]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive oxygen species and the central nervous system. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef]
- Hirsch, E.C. Does oxidative stress participate in nerve cell death in Parkinson’s disease. Eur. Neurol 1993, 33, 52–59. [Google Scholar] [CrossRef]
- Crompton, M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999, 341, 233–249. [Google Scholar] [CrossRef]
- Akao, M.; O’Rourke, B.; Teshima, Y.; Seharaseyon, J.; Marba’n, E. Mechanistically distinct steps in the mitochondrial death pathway triggered by oxidative stress in cardiac myocytes. Circ. Res. 2003, 92, 186–194. [Google Scholar] [CrossRef]
- Tang, X.Q.; Feng, J.Q.; Chen, J.; Chen, P.X.; Zhi, J.L.; Cui, Y.; Guo, R.X.; Yu, H.M. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: Mechanisms via MMP, ROS, and Bcl-2. Brain Res. 2005, 1057, 57–64. [Google Scholar] [CrossRef]
- Gilmore, T.D. The Rel/NF-κB signal transduction pathway: Introduction. Oncogene 1999, 18, 6842–6844. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef]
- Brasier, A.R. The NF-κB regulatory network. Cardiovasc. Toxicol. 2006, 6, 111–130. [Google Scholar] [CrossRef]
- Perkins, N.D. Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef]
- Tian, B.; Brasier, A.R. Identification of a nuclear factor κB-dependent gene network. Rec. Prog. Horm. Res. 2003, 58, 95–130. [Google Scholar] [CrossRef]
- Sen, R.; Baltimore, D. Inducibility of k immunoglobulins enhancer-binding protein NF-κB by a posttranslational mechanism. Cell 1986, 47, 921–929. [Google Scholar] [CrossRef]
- Natarajan, K.; Singh, S.; Burke, T.R., Jr.; Grunberger, D.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar]
- Alnemri, E.S.; Livingston, D.J.; Nicholson, D.W.; Salvesen, G.; Thornberry, N.A.; Wong, W.W.; Yuan, J. Human ICE/CED-3 protease nomenclature. Cell 1996, 87, 171. [Google Scholar] [CrossRef]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell. Death Differ. 1999, 6, 99–104. [Google Scholar]
- Sample Availability: Samples of the compounds 1, 4–7 are available from the author Xiaobin Zeng.
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zeng, X.; Zhou, X.; Cui, L.; Liu, D.; Wu, K.; Li, W.; Huang, R. The Fruits of Wampee Inhibit H2O2-Induced Apoptosis in PC12 Cells via the NF-?B Pathway and Regulation of Cellular Redox Status. Molecules 2014, 19, 7368-7387. https://doi.org/10.3390/molecules19067368
Zeng X, Zhou X, Cui L, Liu D, Wu K, Li W, Huang R. The Fruits of Wampee Inhibit H2O2-Induced Apoptosis in PC12 Cells via the NF-?B Pathway and Regulation of Cellular Redox Status. Molecules. 2014; 19(6):7368-7387. https://doi.org/10.3390/molecules19067368
Chicago/Turabian StyleZeng, Xiaobin, Xin Zhou, Liao Cui, Decheng Liu, Kefeng Wu, Wende Li, and Ren Huang. 2014. "The Fruits of Wampee Inhibit H2O2-Induced Apoptosis in PC12 Cells via the NF-?B Pathway and Regulation of Cellular Redox Status" Molecules 19, no. 6: 7368-7387. https://doi.org/10.3390/molecules19067368
APA StyleZeng, X., Zhou, X., Cui, L., Liu, D., Wu, K., Li, W., & Huang, R. (2014). The Fruits of Wampee Inhibit H2O2-Induced Apoptosis in PC12 Cells via the NF-?B Pathway and Regulation of Cellular Redox Status. Molecules, 19(6), 7368-7387. https://doi.org/10.3390/molecules19067368



