The Use of Supported Acidic Ionic Liquids in Organic Synthesis
Abstract
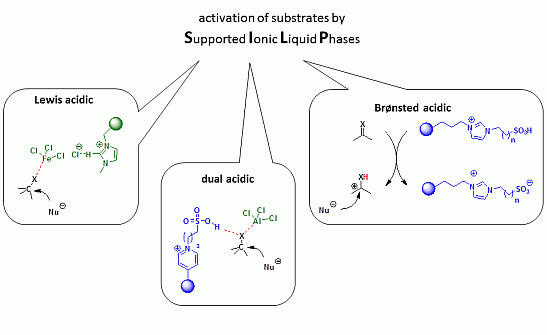
:1. Introduction

2. Preparation and Characterization of Supported Acidic Ionic Liquids
2.1. Immobilisation of Lewis Acidic ILs
| Ionic Liquid | Supporting Method | Support | Catalytic Reaction | Ref. |
|---|---|---|---|---|
| [C4C1im]Cl/AlCl3 | impregnation | silica | alkylation | [20] |
| [C4C1im]Cl/AlCl3 | impregnation | SiO2, Al2O3, TiO2, ZrO2 | Friedel-Crafts alkylation | [21] |
| [C4C1im]Cl/AlCl3 | impregnation | amorphous silica | Friedel-Crafts alkylation | [18] |
| [C4C1im]Cl/AlCl3 | impregnation | glass, activated carbon | alkylation | [22] |
| [C4C1im]Cl/AlCl3 | impregnation | silica gel, MCM-41, SBA-15 | oligomerisation | [22] |
| [C4C1im]Cl/FeCl3 | impregnation | amorphous silica, charcoal | Friedel-Crafts acylation | [23] |
| [(C2)3NH]Cl/AlCl3 | impregnation | molecular sieves | epoxy ether cleavage | [24] |
| [(C2)4N][SnCl5] | impregnation | silica | Prins reaction | [25] |
| [C2C1im]Cl/AlCl3 | impregnation | chemically pretreated silica gel | Friedel-Crafts alkylation | [26] |
| [C4C1im]Cl/AlCl3 | impregnation | amorphous silica | alkylation | [27] |
| [((EtO)3Si)3C3C1im]Cl/AlCl3 | grafting (condensation) | MCM-41 | Friedel-Crafts alkylation | [18] |
| [((EtO)3Si)3C3C1im]Cl/AlCl3 | grafting (condensation) | silica gel | alkylation | [22] |
| [((EtO)3Si)3C3C1im]Cl/AlCl3 | grafting (condensation) | MCM-41 | alkylation | [27] |
| [((EtO)3Si)3C3C1im]Cl/FeCl3 | grafting (condensation) | MCM-41 | Friedel-Crafts alkylation | [28] |
| [((MeO)3Si)3C3pyr][SnCl5] [((EtO)3Si)3C3(C3)3N][SnCl5] | grafting (condensation) | silica | Prins reaction | [25] |
| [((EtO)3Si)3C3((EtO)3Si)3C3im]Cl/InCl3 | co-condensation with tetraethoxysilane | mesoporous silica | Friedel-Crafts alkylation | [29] |
| [(C1=C2)(ClO2S)4C4im][OTf] [(C1=C2)(ClO2S)3C3im][OTf] | grafting (radical chain transfer reaction) | 3-mercaptopropylated silica | esterification, nitration | [30] |
| [(C1=C2)(ClO2S)4C4im][OTf] | grafting (radical chain transfer reaction) | 3-mercaptopropylated silica | conjugate addition | [11] |
| [(C1=C2)(ClO2S)4C4im][OTf] | grafting (radical chain transfer reaction) | 3-mercaptopropylated silica | domino Knoevenagel condensation/Michael addition | [31] |
| [C1Him]Cl/AlCl3 | grafting (alkylation) | Merrifield resin | Knoevenagel condensation | [32] |
| [C1Him][FeCl4] | grafting (alkylation) | chloromethylated polystyrene | cycloaddition of epoxide or azridine with CO2 | [33] |
| [Hpyr]Cl/AlCl3 | grafting (alkylation) | Merrifield resin | Knoevenagel condensation | [34] |
| [C4(C1=C1)im][AlCl4] [C2(C1=C1)im][AlCl4] [C2pyr][AlCl4] [C4pyr][AlCl4] | polymerisation and alkylation | polymer | Diels-Alder reaction | [35] |
| [C4(C1=C1)im]Cl/GaCl3 | copolymerization | polymer (styrene, [C4(C1=C1)im]) | acetal formation | [36] |
| [C4(C1=C1)im][ZnCl2Br2] [C2(C1=C1)im][ZnCl2Br2] | polymerisation | polymer | cycloaddition of epoxide with CO2 | [37] |
2.1.1. Immobilisation of Lewis Acidic ILs on Inorganic Supports






2.1.2. Immobilisation of Lewis Acidic ILs on Organic Supports



2.2. Immobilisation of Brønsted Acidic ILs
| Ionic Liquid | Supporting Method | Support | Catalytic Reaction | Ref. |
|---|---|---|---|---|
| [NH4][H2PO4] | impregnation | alumina | multicomponent condensation | [38] |
| [(HSO3)4C4pyr][HSO4] | impregnation | silica (Aerosil 300) | cyclocondensation | [39] |
| [(HSO3)4C4pyr][HSO4] | impregnation | silica (Aerosil 300) | aldol condensation | [40] |
| [C1(HSO3)4C4im][OTf] [C4(HSO3)4C4im][OTf] [C1(HSO3)4C4im][HSO4] [C4(HSO3)4C4im][HSO4] | impregnation | silica gel | oligomerisation | [41] |
| [C1(HSO3)4C4im][OTf] | impregnation | silica gel | oligomerisation | [42] |
| [C1(HSO3)3C3im][OTf] | ion exchange | montmorillonite clay | transesterifcation | [43] |
| [(HO2C)C1C1im][BF4] [(HO2C)C1C1im]Cl [(HO2C)C1pyr]Cl | sol-gel | silica | deoximation | [44] |
| [((EtO)3Si)3C3C1im][HSO4] | grafting (condensation) | silica material (obtained from TEOS) | Baeyer-Villiger oxidation | [45] |
| [((EtO)3Si)3C3C1im][HSO4] | grafting (condensation) | silica material (obtained from TEOS) | multicomponent condensation | [46,47,48,49,50] |
| [((EtO)3Si)3C3C1im][HSO4] | grafting (condensation) | magnetic nanoparticles | multicomponent condensation (Biginelli reaction) | [51] |
| [((EtO)3Si)3C3(HSO3)3C3im]Cl | grafting (condensation) | silica | hydrolysis | [52] |
| [((EtO)3Si)3C3(HSO3)3C3bim]Cl | grafting (condensation) | silica gel | transesterification | [53] |
| [((EtO)3Si)3C3(HSO3)3C3bim]Cl | grafting (condensation) | silica gel | multicomponent condensation | [54] |
| [((EtO)3Si)3C3(HSO3)4C4im][HSO4] | grafting (condensation) | silica gel | multicomponent condensation | [55] |
| [((EtO)3Si)3C3(HSO3)4C4im][HSO4] | grafting (condensation) | nano-silica (Cabosil 20) | multicomponent condensation | [56] |
| [((EtO)3Si)3C3(HSO3)4C4im][HSO4] | grafting (condensation) | magnetic nanoparticles coated with silica | multicomponent condensation | [57,58] |
| [((HO3S)C6H4)3((EtO)3Si)3C3P]Cl | grafting (condensation) | magnetic nanoparticles | acetal formation | [59] |
| [(C1=C2)(HSO3)4C4im][OTf] [(C1=C2)(HSO3)3C3im][OTf] | grafting (radical chain transfer reaction) | 3-mercaptopropylated silica | esterification, nitration | [30] |
| [(C1=C2)(HSO3)4C4im][OTf] | grafting (radical chain transfer reaction) | 3-mercaptopropylated silica | conjugate addition | [11] |
| [(C1=C2)(HSO3)4C4im][OTf] | grafting (radical chain transfer reaction) | 3-mercaptopropylated silica | domino Knoevenagel condensation/Michael addition | [31] |
| [(HSO3)3C3(C1=C1)im][HSO4] | grafting (radical chain transfer reaction) | 3-mercaptopropylated silica | esterification | [60] |
| [(C1=C2)(HSO3)4C4im][OTf] | grafting (radical chain transfer reaction) | 3-mercaptopropylated magnetic mesoporous silica | esterification | [61] |
| [((EtO)3Si)3C3(HSO3)3C3im][HSO4] | sol-gel method | silica (obtained from TEOS) | acetal formation | [62] |
| [FcC1Him][HSO4] | grafting (alkylation) | Merrifield resin | allylation of aldehydes | [63] |
| [(HSO3)3C3Him][HSO4] | grafting (alkylation) | Merrifield resin | esterification | [64] |
| [(HSO3)3C3Him][ HSO4] | grafting (alkylation) | chloromethylated polystyrene | nitration | [65] |
| [(HSO3)3C3Him][HSO4] | grafting (alkylation) | silica polystyrene hybrid | esterification | [66] |
| [(HSO3)4C4pyr][HSO4] | grafting alkylation | poly(4-vinylpyridine) | domino Knoevenagel condensation/Michael reaction | [67] |
| [(HSO3)4C4pyr][HSO4] | grafting alkylation | poly(4-vinylpyridine) | multicomponent reaction | [68] |
| [[(HSO3)4C4melamine][HSO4] | grafting (alkylation) | melamine-formaldehyde resin | acetal formation | [69] |
| [(HSO3)3C3pyr]/[PW12O40] | polymerisation | poly(4-vinylpyridine) | cyclocondensation | [70] |
| [(HSO3)4C4(C1=C1)im][OTf] | copolymerisation | polymer (styrene, [(HSO3)4C4(C1=C1)im]) | acetal formation | [71] |
| [(HSO3)4C4(C1=C1)im][HSO4] | copolymerisation | polymer | muticomponent condensation | [72] |
| [((EtO)3Si)3C3C4im][HSO4] | grafting (condensation) | cellulose | multicomponent condensation | [73] |
2.2.1. Immobilisation of Brønsted Acidic ILs on Inorganic Supports




2.2.2. Immobilisation of Brønsted Acidic ILs on Organic Supports


2.3. Immobilisation of Dual Acidic ILs with Brønsted Acidic and Lewis Acidic Sites
| Ionic Liquid | Supporting Method | Support | Catalytic Reaction | Ref. |
|---|---|---|---|---|
| [((EtO)3Si)3C3(HSO3)3C3im][HSO4] | grafting (condensation) | Fe-incorporated SBA-15 | esterification | [74] |
| [(HSO3)4C4pyr]Cl/AlCl3 | grafting (alkylation | poly(4-vinylpyridine) | domino Knoevenagel-type condensation/Michael reaction | [75] |
| [[(HSO3)4C4melamine][HSO4]/CuI | grafting (alkylation) | melamine-formaldehyde resin | acetal formation | [76] |

3. Catalytic Reactions in the Presence of Acidic SILPs


3.1. Friedel-Crafts Reactions

3.2. Nitration

3.3. Alkylation and Oligomerisation
3.4. Esterification and Transesterification

3.5. Acetal Formation

3.6. Cleavage or Cycloaddition Reactions of epoxides


3.7. Condensation Reactions





3.8. Multicomponent Reactions












3.9. Miscellaneous Reactions

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wilson, K.; Clark, J.H. Solid acids and their use as environmentally friendly catalysts in organic synthesis. Pure Appl. Chem. 2000, 72, 1313–1319. [Google Scholar]
- Estager, J.; Holbrey, J.D.; Swadźba-Kwaśny, M. Halometallate ionic liquids—Revisited. Chem. Soc. Rev. 2014, 43, 847–886. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F. Acidic bronsted ionic liquids. Org. Prep. Proc. Int. 2010, 42, 285–362. [Google Scholar] [CrossRef]
- Lin, I.J.B.; Vasam, C.S. Metal-containing ionic liquids and ionic liquid crystals based on imidazolium moiety. J. Organomet. Chem. 2005, 690, 3498–3512. [Google Scholar] [CrossRef]
- Chiappe, C.; Rajamani, S. Structural effects on the physico-chemical and catalytic properties of acidic ionic liquids: An overview. Eur. J. Org. Chem. 2011, 28, 5517–5539. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Cho, C.W.; Yun, Y.S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- Zhao, D.B.; Wu, M.; Kou, Y.; Min, E.Z. Ionic liquids: Applications in catalysis. Catal. Today 2002, 74, 157–189. [Google Scholar] [CrossRef]
- Qiao, Y.; Headley, A.D. Ionic liquid immobilized organocatalysts for asymmetric reactions in aqueous media. Catalysts 2013, 3, 709–725. [Google Scholar]
- Liu, Y.; Wang, S.S.; Liu, W.; Wan, Q.X.; Wu, H.H.; Gao, G.H. Transition-metal catalyzed carbon-carbon couplings mediated with functionalized ionic liquids, supported-ionic liquid phase, or ionic liquid media. Curr. Org. Chem. 2009, 13, 1322–1346. [Google Scholar] [CrossRef]
- Hagiwara, H.; Sekifuji, M.; Hoshi, T.; Suzuki, T.; Quanxi, B.; Qiao, K.; Yokoyama, C. Sustainable conjugate addition of indoles catalyzed by acidic ionic liquid immobilized on silica. Synlett 2008, 39, 608–610. [Google Scholar]
- Cui, X.; Zhang, S.; Shi, F.; Ma, X.; Lu, L.; Deng, Y. The influence of the acidity of ionic liquids on catalysis. ChemSusChem 2010, 3, 1043–1047. [Google Scholar] [CrossRef]
- Contreras, R.; Aizman, A.; Tapia, R.A.; Cerda-Monje, A. Lewis molecular acidity of ionic liquids from empirical energy-density models. J. Phys. Chem. B 2013, 117, 1911–1920. [Google Scholar] [CrossRef]
- Cerda-Monje, A.; Ormazábal-Toledo, R.; Cárdenas, C.; Fuentealba, P.; Contreras, R. Regional electrophilic and nucleophilic fukui functions efficiently highlight the lewis acidic/basic regions in ionic liquids. J. Phys. Chem. B 2014, 118, 3696–3701. [Google Scholar] [CrossRef]
- Riisager, A.; Fehrmann, R.; Haumann, M.; Wasserscheid, P. Supported ionic liquids: Versatile reaction and separation media. Top. Catal. 2006, 40, 91–102. [Google Scholar]
- Mehnert, C.P.; Cook, R.A.; Dispenziere, N.C.; Afeworki, M. Supported ionic liquid catalysis—A new concept for homogeneous hydroformylation catalysis. J. Am. Chem. Soc. 2002, 124, 12932–12933. [Google Scholar] [CrossRef]
- Virtanen, P.; Salmi, T.O.; Mikkola, J.P. Supported Ionic Liquid Catalysts (SILCA) for preparation of organic chemicals. Top. Catal. 2010, 53, 1096–1103. [Google Scholar] [CrossRef]
- Valkenberg, M.H.; deCastro, C.; Hölderich, W.F. Immobilisation of chloroaluminate ionic liquids on silica materials. Top. Catal. 2001, 14, 139–144. [Google Scholar] [CrossRef]
- Andanson, J.M.; Baiker, A. Interactions of 1-ethyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid with alumina nanoparticles and organic solvents studied by infrared spectroscopy. J. Phys. Chem. C 2013, 117, 12210–12217. [Google Scholar] [CrossRef]
- Benazzi, E.; Hirschauer, A.; Joly, J.F.; Olivier, H.; Berhard, J.Y. Catalyseur D’alkylation de Paraffines. EP0553009, 1993. [Google Scholar]
- DeCastro, C.; Sauvage, E.; Valkenberg, M.H.; Hölderich, W.F. Immobilised Ionic Liquids as Lewis Acid Catalysts for the Alkylation of Aromatic Compounds with Dodecene. J. Catal. 2000, 196, 86–94. [Google Scholar] [CrossRef]
- Liu, S.; Shang, J.; Zhang, S.; Yang, B.; Deng, Y. Highly efficient trimerization of isobutene over silica supported chloroaluminate ionic liquid using C4 feed. Catal. Today 2013, 200, 41–48. [Google Scholar] [CrossRef]
- Valkenberg, M.H.; deCastro, C.; Hölderich, W.F. Friedel-Crafts acylation of aromatics catalysed by supported ionic liquids. Appl. Catal. A 2001, 215, 185–190. [Google Scholar] [CrossRef]
- Zhi, H.Z.; Shi, H.L.; Hu, Y.; Xia, K.D.; Zhang, P.; Yang, J.F. Epoxy ether cleaving reactions catalyzed by supporting Lewis acidic ionic liquid. Chin. Chem. Lett. 2012, 23, 1217–1220. [Google Scholar] [CrossRef]
- Jyothi, T.M.; Kaliya, M.L.; Landau, M.V. A Lewis acidic catalyst anchored on silica grafted with quternary alkylammonium chloride moieties. Angew. Chem. Int. Ed. 2001, 40, 2881–2884. [Google Scholar]
- Joni, J.; Haumann, M.; Wasserscheid, P. Development of a supported ionic liquid phase (silp) catalyst for slurry-phase Friedel–Crafts alkylations of cumene. Adv. Synth. Catal. 2009, 351, 423–431. [Google Scholar] [CrossRef]
- Kumar, P.; Vermeiren, W.; Dath, J.P.; Hölderich, W.F. Production of alkylated gasoline using ionic liquids and immobilized ionic liquids. Appl. Catal. A 2006, 304, 131–141. [Google Scholar] [CrossRef]
- Wang, G.; Yu, N.; Peng, L.; Tan, R.; Zhao, H.; Yin, D.; Qiu, H.; Fu, Z.; Yin, D. Immobilized chloroferrate ionic liquid: An efficient and reusable catalyst for synthesis of diphenylmethane and its derivatives. Catal. Lett. 2008, 123, 252–258. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, N.; Wang, J.; Zhuang, D.; Ding, Y.; Tan, R.; Yin, D. Preparation and catalytic activity of periodic mesoporous organosilica incorporating Lewis acidic chloroindate(III) ionic liquid moieties. Microporous Mesoporous Mater. 2009, 122, 240–246. [Google Scholar] [CrossRef]
- Qiao, K.; Hagiwara, H.; Yokoyama, C. Acidic ionic liquid modified silica gel as novel solid catalysts for esterification and nitration reactions. J. Mol. Catal. A Chem. 2006, 246, 65–69. [Google Scholar] [CrossRef]
- Hagiwara, H.; Sekifuji, M.; Hoshi, T.; Qiao, K.; Yokoyama, C. Synthesis of bis(indolyl)methanes catalyzed by acidic ionic liquid immobilized on silica (ILIS). Synlett 2007, 8, 1320–1322. [Google Scholar]
- Parvanak Boroujeni, P.; Jafarinasab, M. Polystyrene-supported chloroaluminate ionic liquid as a new heterogeneous Lewis acid catalyst for Knoevenagel condensation. Chin. Chem. Lett. 2012, 23, 1067–1070. [Google Scholar] [CrossRef]
- Gao, J.; Song, Q.W.; He, L.N.; Liu, C.; Yang, Z.Z.; Han, X.; Li, X.D.; Song, Q.C. Preparation of polystyrene-supported Lewis acidic Fe(III) ionic liquid and its application in catalytic conversion of carbon dioxide. Tetrahedron 2012, 68, 3835–3842. [Google Scholar] [CrossRef]
- Parvanak Boroujeni, P.; Jafarinasab, M. Polystyrene-supported pyridinium chloroaluminate ionic liquid as a new heterogeneous Lewis acid catalyst for Knoevenagel condensation. J. Chem. Res. 2012, 36, 429–431. [Google Scholar] [CrossRef]
- Bae, H.W.; Han, J.S.; Jung, S.; Cheong, M.; Kim, H.S.; Lee, J.S. Polymer-supported chloroaluminate catalysts for the Diels–Alder reaction of cyclopentadiene with methyl methacrylate. Appl. Catal. A 2007, 331, 34–38. [Google Scholar] [CrossRef]
- Bao, Q.; Qiao, K.; Tomida, D.; Yokoyama, C. Acetalization of carbonyl compounds catalyzed by GaCl3 immobilized on imidazolium-styrene copolymers. Catal. Commun. 2009, 10, 1625–1628. [Google Scholar] [CrossRef]
- Lee, B.; Ko, N.H.; Ahn, B.S.; Cheong, M.; Kim, H.S.; Lee, J.S. Polymer-supported zinc tetrahalide catalysts for the coupling reactions of CO2 and epoxides. Bull. Korean Chem. Soc. 2007, 28, 2025–2028. [Google Scholar] [CrossRef]
- Emrani, A.; Davoodnia, A.; Tavakoli-Hoseini, N. Alumina supported ammonium dihydrogenphosphate (NH4H2PO4/Al2O3): Preparation, characterization and its application as catalyst in the synthesis of 1,2,4,5-tetrasubstituted imidazoles. Bull. Korean Chem. Soc. 2011, 32, 2385–2390. [Google Scholar] [CrossRef]
- Yassaghi, G.; Davoodnia, A.; Allameh, S.; AZare-Bidaki, A.; Tavakoli-Hoseini, N. Preparation, characterization and first application of aerosil silica supported acidic ionic liquid as a reusable heterogeneous catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Bull. Korean Chem. Soc. 2012, 33, 2724–2730. [Google Scholar] [CrossRef]
- Davoodnia, A.; Yassaghi, G. Solvent-free selective cross-aldol condensation of ketones with aromatic aldehydes efficiently catalyzed by a reusable supported acidic ionic liquid. Chin. J. Catal. 2012, 33, 1950–1957. [Google Scholar] [CrossRef]
- Fehér, C.; Kriván, E.; Hancsók, J.; Skoda-Földes, R. Oligomerisation of isobutene with silica supported ionic liquid catalysts. Green Chem. 2012, 14, 403–409. [Google Scholar] [CrossRef]
- Fehér, C.; Kriván, E.; Kovács, J.; Hancsók, J.; Skoda-Földes, R. Support effect on the catalytic activity and selectivity of SILP catalysts in isobutene trimerization. J. Mol. Catal. A Chem. 2013, 372, 51–57. [Google Scholar] [CrossRef]
- Ratti, R.; Kaur, S.; Vaultier, M.; Singh, V. Preparation, characterization and catalytic activity of MMT-clay exchanged sulphonic acid functionalized ionic liquid for transesterification of β-ketoesters. Catal.Commun. 2010, 11, 503–507. [Google Scholar]
- Li, D.; Shi, F.; Guo, S.; Deng, Y. One-pot synthesis of silica gel confined functional ionic liquids: Effective catalysts for deoximation under mild conditions. Tetrahedron Lett. 2004, 45, 265–268. [Google Scholar] [CrossRef]
- Chrobok, A.; Baj, S.; Pudło, W.; Jarzębski, A. Supported hydrogensulfate ionic liquid catalysis in Baeyer–Villiger reaction. Appl. Catal. A 2009, 366, 22–28. [Google Scholar] [CrossRef]
- Damavandi, S. Immobilized ionic liquid-catalyzed synthesis of pyrano[3,2-b]indole derivatives. E-J. Chem. 2012, 9, 1490–1493. [Google Scholar] [CrossRef]
- Sandaroos, R.; Damavandi, S.; Salimi, M. Facile one-pot synthesis of 5-amino-7-aryl-6-cyano-4Hpyrano[3,2-b]pyrroles using supported hydrogen sulfate ionic liquid. Monatsh. Chem. 2012, 143, 1655–1661. [Google Scholar] [CrossRef]
- Damavandi, S.; Sandaroos, R. Novel synthetic route to pyrano[2,3-b]pyrrole derivatives. Synth. React. Inorg. Met. Org. Chem. 2012, 42, 621–627. [Google Scholar]
- Damavandi, S.; Sandaroos, R. Novel multicomponent synthesis of 2,9-dihydro-9-methyl-2-oxo-4-aryl- 1H-pyrido[2,3-b]indole-3-carbonitrile compounds. J. Chem. Sci. 2013, 125, 95–100. [Google Scholar] [CrossRef]
- Goldani, M.T.; Sandaroos, R.; Damavandi, S. Efficient polymeric catalyst for one-pot synthesis of acenaphtho[1,2-b]pyrroles. Res. Chem. Intermed. 2014, 40, 139–147. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. Brønsted acidic ionic liquid based magnetic nanoparticles: A new promoter for the Biginelli synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones. New J. Chem. 2014, 38, 358–365. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Owereh, O.S. Synthesis of a sulfonic acid functionalized acidic ionic liquid modified silica catalyst and applications in the hydrolysis of cellulose. Catal. Commun. 2010, 11, 1072–1075. [Google Scholar] [CrossRef]
- Kotadia, D.A.; Soni, S.S. Sulfonic acid functionalized solid acid: An alternative eco-friendly approach for transesterification of non-edible oils with high free fatty acids. Monatsh. Chem. 2013, 144, 1735–1741. [Google Scholar] [CrossRef]
- Kotadia, D.A.; Soni, S.S. Silica gel supported –SO3H functionalised benzimidazolium based ionic liquid as a mild and effective catalyst for rapid synthesis of 1-amidoalkyl naphthols. J. Mol. Catal. A Chem. 2012, 353–354, 44–49. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wie, Y. A silica gel supported dual acidic ionic liquid: An efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkyl naphthols. Green Chem. 2010, 12, 2246–2254. [Google Scholar] [CrossRef]
- Safaei, S.; Mohammadpoor-Baltork, I.; Khosropour, A.R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Nano-silica supported acidic ionic liquid as an efficient catalyst for the multi-component synthesis of indazolophthalazine-triones and bis-indazolophthalazine-triones. Catal. Sci. Technol. 2013, 3, 2717–2722. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, H.; Luo, J.; Wie, Y. A magnetic nanoparticle supported dual acidic ionic liquid: A “quasi-homogeneous” catalyst for the one-pot synthesis of benzoxanthenes. Green Chem. 2012, 14, 201–208. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Mohammadi, S. Magnetic, acidic, ionic liquid-catalyzed one-pot synthesis of spirooxindoles. ACS Comb. Sci. 2013, 15, 512–518. [Google Scholar] [CrossRef]
- Wang, P.; Kong, A.G.; Wang, W.J.; Zhu, H.Y.; Shan, Y.K. Facile preparation of ionic liquid functionalized magnetic nano-solid acid catalysts for acetalization reaction. Catal. Lett. 2010, 135, 159–164. [Google Scholar] [CrossRef]
- Miao, J.; Wan, H.; Guan, G. Synthesis of immobilized Brønsted acidic ionic liquid on silica gel as heterogeneous catalyst for esterification. Catal. Commun. 2011, 12, 353–356. [Google Scholar] [CrossRef]
- Zhen, B.; Jiao, Q.; Zhang, Y.; Wu, Q.; Li, H. Acidic ionic liquid immobilized on magnetic mesoporous silica: Preparation and catalytic performance in esterification. Appl. Catal. A 2012, 445–446, 239–245. [Google Scholar]
- Miao, J.; Wan, H.; Shao, Y.; Guan, G.; Xu, B. Acetalization of carbonyl compounds catalyzed by acidic ionic liquid immobilized on silica gel. J. Mol. Catal. A Chem. 2011, 348, 77–82. [Google Scholar] [CrossRef]
- Rashinkar, G.; Kamble, S.; Kumbhar, A.; Salunkhe, R. An expeditious synthesis of homoallylic alcohols using Brønsted acidic supported ionic liquid phase catalyst with pendant ferrocenyl group. Catal. Commun. 2011, 12, 1442–1447. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, H.; Miao, J.; Han, M.; Yang, C.; Guan, G. Reusable and efficient polystyrene-supported acidic ionic liquid catalyst for esterifications. J. Mol. Catal. A Chem. 2010, 332, 152–157. [Google Scholar] [CrossRef]
- Li, L.X.; Ling, Q.L.; Liu, Z.L.; Xing, X.D.; Zhu, X.Q.; Meng, X. Reusable and efficient polystryrene-supported acidic ionic liquid catalyst for mononitration of aromatic compounds. Bull. Korean Chem. Soc. 2012, 33, 3373–3377. [Google Scholar] [CrossRef]
- Shao, Y.; Wan, H.; Miao, J.; Guan, G. Synthesis of an immobilized Brønsted acidic ionic liquid catalyst on chloromethyl polystyrene grafted silica gel for esterification. React. Kinet. Mech. Catal. 2013, 109, 149–158. [Google Scholar] [CrossRef]
- Parvanak Boroujeni, K.; Shojaei, P. Poly(4-vinylpyridine)-supported dual acidic ionic liquid: An environmentally friendly heterogeneous catalyst for the one-pot synthesis of 4,4'-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols). Turk. J. Chem. 2013, 37, 756–764. [Google Scholar] [CrossRef]
- Parvanak Boroujeni, P.; Taheri, S.; Seyfipour, G. Poly(4-vinylpyridine)-supported dual acidic ionic liquid: A novel heterogeneous catalyst for the synthesis of β-acetamido ketones. Synth. React. Inorg. Met. Org. Chem. 2014, 44, 84–88. [Google Scholar] [CrossRef]
- Xing, G. Synthesis of a novel melamine-formaldehyde resin-supported ionic liquid with Brønsted acid sites and its catalytic activities. Monatshefte Chem. 2013, 144, 1369–1374. [Google Scholar] [CrossRef]
- Wang, J.; Zong, Y.; Fu, R.; Niu, Y.; Yue, G.; Quan, Z.; Wang, X.; Pan, Y. Poly(4-vinylpyridine) supported acidic ionic liquid: A novel solid catalyst for the efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones under ultrasonic irradiation. Ultrason. Sonochem. 2014, 21, 29–34. [Google Scholar] [CrossRef]
- Sugimura, R.; Qiao, K.; Tomida, D.; Yokoyama, C. Immobilization of acidic ionic liquids by copolymerization with styrene and their catalytic use for acetal formation. Catal. Commun. 2007, 8, 770–772. [Google Scholar] [CrossRef]
- Jahanbin, B.; Davoodnia, A.; Behmadi, H.; Tavakoli-Hoseini, N. Polymer support immobilized acidic ionic liquid: Preparation and its application as catalyst in the synthesis of Hantzsch 1,4-dihydropyridines. Bull. Korean Chem. Soc. 2012, 33, 2140–2144. [Google Scholar] [CrossRef]
- Satasia, S.P.; Kalaria, P.N.; Raval, D.K. Acidic ionic liquid immobilized on cellulose: An efficient and recyclable heterogeneous catalyst for the solventfree synthesis of hydroxylated trisubstituted pyridines. RSC Adv. 2013, 3, 3184–3188. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, Y.; Zhang, C.; Wang, L.; Wan, H.; Guan, G. Biodiesel production by esterification of oleic acid over brønsted acidic ionic liquid supported onto Fe-incorporated SBA-15. Ind. Eng. Chem. Res. 2012, 51, 16590–16596. [Google Scholar] [CrossRef]
- Parvanak Boroujeni, P.; Ghasemi, P. Synthesis and application of a novel strong and stable supported ionic liquid catalyst with both Lewis and Brønsted acid sites. Catal. Commun. 2013, 37, 50–54. [Google Scholar] [CrossRef]
- Du, Y.; Shao, L.; Luo, L.; Shi, S.; Qi, C. Preparation of a novel solid acid catalyst with Lewis and Br_nsted acid sites and its application in acetalization. Turk. J. Chem. 2014, 38, 157–163. [Google Scholar]
- Joni, J.; Haumann, M.; Wasserscheid, P. Continuous gas-phase isopropylation of toluene and cumene using highly acidic Supported Ionic Liquid Phase (SILP) catalysts. Appl. Catal. A 2010, 372, 8–15. [Google Scholar] [CrossRef]
- Sun, J.; Fujita, S.; Arai, M. Development in the green synthesis of cyclic carbonate from carbon dioxide using ionic liquids. J. Organomet. Chem. 2005, 690, 3490–3497. [Google Scholar] [CrossRef]
- Munshi, K.M.; Lomate, S.T.; Deshpande, R.M.; Rane, V.H.; Kelkar, A.A. Synthesis of acrolein by gas-phase dehydration of glycerol over silica supported Bronsted acidic ionic liquid catalysts. J. Chem. Technol. Biotechnol. 2010, 85, 1319–1324. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoda-Földes, R. The Use of Supported Acidic Ionic Liquids in Organic Synthesis. Molecules 2014, 19, 8840-8884. https://doi.org/10.3390/molecules19078840
Skoda-Földes R. The Use of Supported Acidic Ionic Liquids in Organic Synthesis. Molecules. 2014; 19(7):8840-8884. https://doi.org/10.3390/molecules19078840
Chicago/Turabian StyleSkoda-Földes, Rita. 2014. "The Use of Supported Acidic Ionic Liquids in Organic Synthesis" Molecules 19, no. 7: 8840-8884. https://doi.org/10.3390/molecules19078840
APA StyleSkoda-Földes, R. (2014). The Use of Supported Acidic Ionic Liquids in Organic Synthesis. Molecules, 19(7), 8840-8884. https://doi.org/10.3390/molecules19078840