New Thiosemicarbazides and 1,2,4-Triazolethiones Derived from 2-(Ethylsulfanyl) Benzohydrazide as Potent Antioxidants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Spectroscopic Characterization


2.2. Antioxidant Activities
2.2.1. DPPH Free Radical Scavenging Activity
| Compound | Structure | Yield (%) | DPPH a (IC50 b µg/mL) | FRAP a Values |
|---|---|---|---|---|
| 2 | 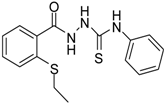 | 96 | 1.08 ± 0.02 | 1193.33 ± 0.05 |
| 3 | 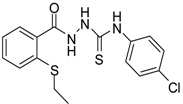 | 93 | 0.22 ± 0.01 | 3054.44 ± 0.15 |
| 4 | 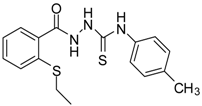 | 95 | 2.91 ± 0.05 | 418.16 ± 0.16 |
| 5 | 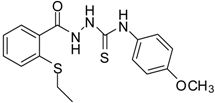 | 94 | 2.69 ± 0.21 | 427.83 ± 0.11 |
| 6 |  | 93 | 4.50 ± 0.01 | 374.44 ± 0.14 |
| 7 | 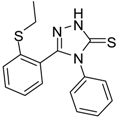 | 75 | 0.74 ± 0.15 | 760.00 ± 0.03 |
| 8 | 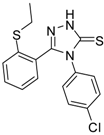 | 73 | 5.64 ± 0.03 | 135.72 ± 0.12 |
| 9 | 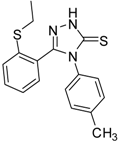 | 70 | 1.51 ± 0.08 | 278.89 ± 0.12 |
| 10 | 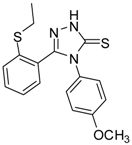 | 75 | 6.30 ± 0.13 | 109.72 ± 0.05 |
| 11 |  | 69 | 3.75 ± 0.01 | 41.81 ± 0.10 |
| Quercetin | 2.54 ± 0.07 | 1371.11 ± 0.26 | ||
| BHT | 18.71 ± 0.01 | 77.83 ± 0.08 | ||
| Trolox | 5.35 ± 0.64 | 987.78 ± 0.14 | ||
| Rutin | 5.25 ± 0.01 | 393.89 ± 0.02 | ||
| Gallic acid (GA) | 1.20 ± 0.13 | 2957.78 ± 0.05 | ||
| Ascorbic acid (AA) | 7.52 ± 0.08 | 1206.67 ± 0.02 |


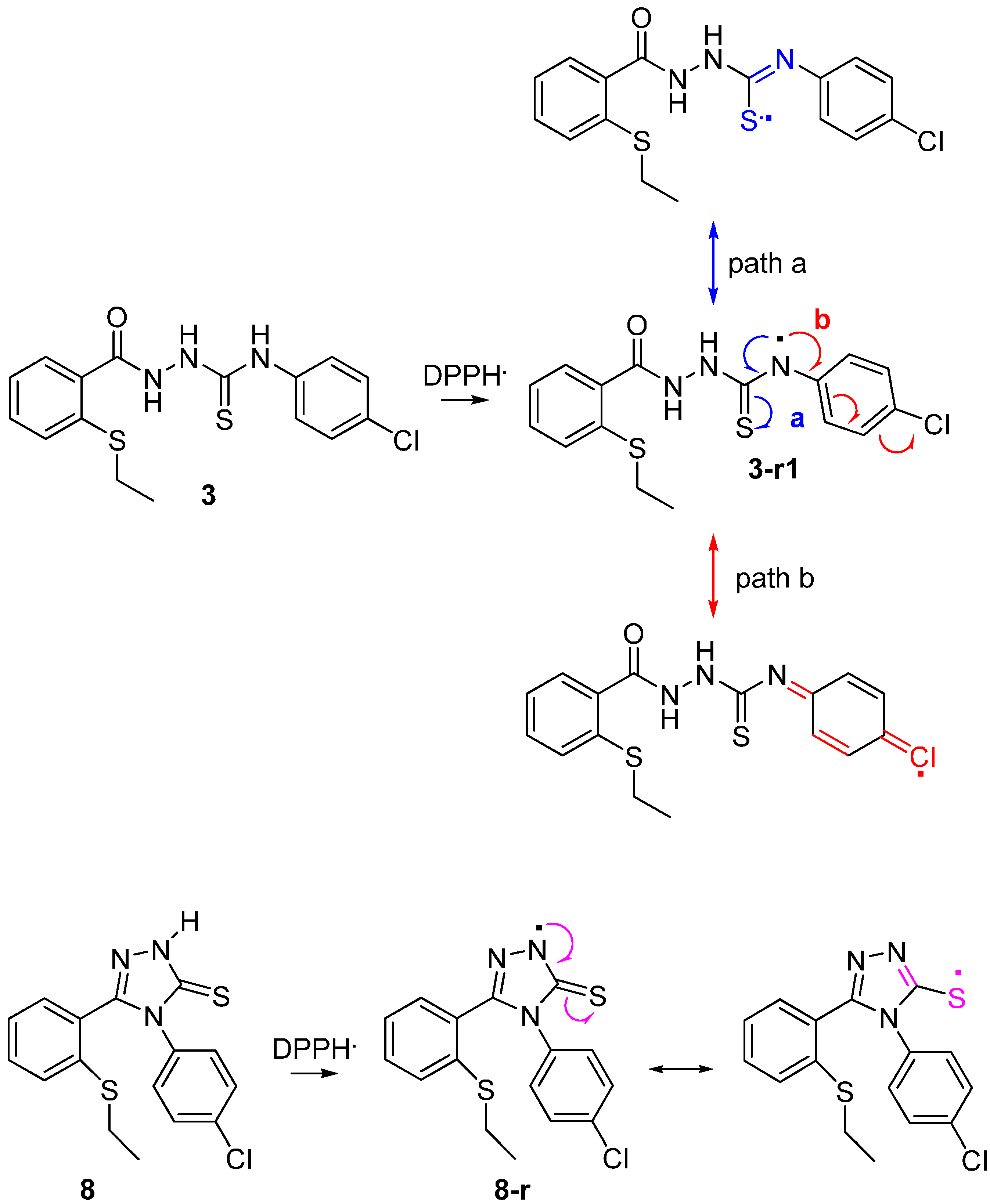
2.2.2. Ferric ions (Fe+3) Reducing Antioxidant Power (FRAP) Assay

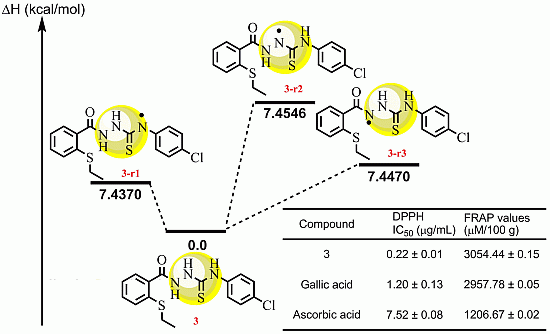
2.2.3 Quantum Calculation of the Antioxidant Activities of Compounds 3 and 8

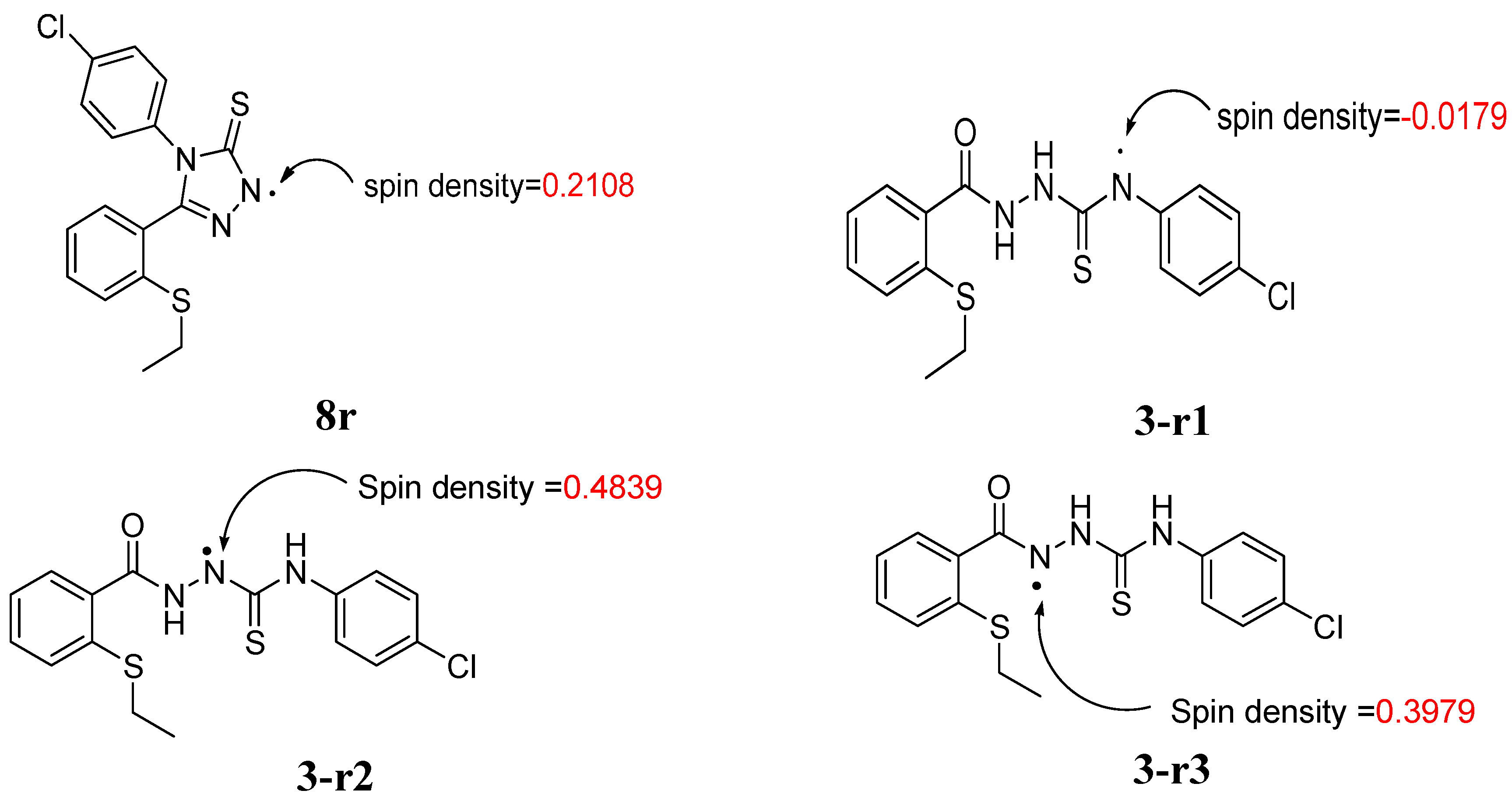


| Compd | ΔHrxn (kcal/mol) | ΔGrxn (kcal/mol) | BDH298 (kcal/mol) |
|---|---|---|---|
| 3r1 | 7.4370 | 8.9157 | 414.8133 |
| 3r2 | 7.4546 | 8.9520 | 426.4630 |
| 3r3 | 7.4470 | 7.3645 | 436.3319 |
| 8r | 8.4776 | 7.9895 | 425.7250 |
3. Experimental
3.1. Chemistry
3.1.1. General Information

3.1.2. General Procedure for the Synthesis of 1-[(2-Ethylsulphanylphenyl)carbonyl]-4-substituted Thiosemicarbazide Derivatives 2–6
3.1.3. General Procedure for the Synthesis of 5-[2-(Ethylsulfanyl)phenyl]-4-substituted-2,4-dihydro-3H-1,2,4-triazole-3-thiones 7–11
3.2. Single Crystal X-ray Structure Determination
3.3. Pharmacological Assays
3.3.1. DPPH Free Radical Scavenging Activity
3.3.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.3.3. Computational Studies
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, W.-L.; Huang, J.-Y.; Shyur, L.-F. Phytoagents for cancer management: Regulation of nucleic acid oxidation, ros, and related mechanisms. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef]
- Grimm, S.; Höhn, A.; Grune, T. Oxidative protein damage and the proteasome. Amino Acids 2012, 42, 23–38. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Kadenbach, B.; Ramzan, R.; Vogt, S. Degenerative diseases, oxidative stress and cytochrome c oxidase function. Trends Mol. Med. 2009, 15, 139–147. [Google Scholar] [CrossRef]
- Diplock, A.T.; Charleux, J.-L.; Crozier-Willi, G.; Kok, F.J.; Rice-Evance, C.; Roberfroid, M.; Stahl, W.; Viña-Ribes, J. Functional food science and defence against reactive oxidative species. Br. J. Nutr. 1998, 80, S77–S112. [Google Scholar]
- Singh, N.; Rajini, P.S. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004, 85, 611–616. [Google Scholar] [CrossRef]
- Jaishree, V.; Ramdas, N.; Sachin, J.; Ramesh, B. In vitro antioxidant properties of new thiazole derivatives. J. Saudi Chem. Soc. 2012, 16, 371–376. [Google Scholar] [CrossRef]
- Ani, V.; Naidu, K. Antioxidant potential of bitter cumin (centratherum anthelminticum (l.) kuntze) seeds in in vitro models. BMC Complem. Altern. M. 2011, 11, 40. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Zhao, X.; Liang, Y.; He, H.; Fu, L. Synthesis and antitumor activity of novel quinazoline derivatives containing thiosemicarbazide moiety. Eur. J. Med. Chem. 2012, 54, 925–930. [Google Scholar] [CrossRef]
- Ezabadi, I.R.; Camoutsis, C.; Zoumpoulakis, P.; Geronikaki, A.; Soković, M.; Glamočilija, J.; Ćirić, A. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg. Med. Chem. 2008, 16, 1150–1161. [Google Scholar] [CrossRef]
- Revelant, G.; Gadais, C.; Mathieu, V.; Kirsch, G.; Hesse, S. Synthesis and antiproliferative studies of 5-aryl-2-(3-thienylamino)-1,3,4-thiadiazoles. Bioorg. Med. Chem. Lett. 2014, 24, 2724–2727. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Le, T.H.; Bui, T.T.T. Antioxidant activities of thiosemicarbazones from substituted benzaldehydes and n-(tetra-o-acetyl-î²-d-galactopyranosyl)thiosemicarbazide. Eur. J. Med. Chem. 2013, 60, 199–207. [Google Scholar] [CrossRef]
- Amorati, R.; Fumo, M.G.; Menichetti, S.; Mugnaini, V.; Pedulli, G.F. Electronic and hydrogen bonding effects on the chain-breaking activity of sulfur-containing phenolic antioxidants. J. Org. Chem. 2006, 71, 6325–6332. [Google Scholar] [CrossRef]
- Tozkoparan, B.; Gökhan, N.; Aktay, G.; Yeşilada, E.; Ertan, M. 6-benzylidenethiazolo[3,2-b]-1,2,4-triazole-5(6h)-onessubstituted with ibuprofen: Synthesis, characterizationand evaluation of anti-inflammatory activity. Eur. J. Med. Chem. 2000, 35, 743–750. [Google Scholar] [CrossRef]
- Varvaresou, A.; Siatra-Papastaikoudi, T.; Tsotinis, A.; Tsantili-Kakoulidou, A.; Vamvakides, A. Synthesis, lipophilicity and biological evaluation of indole-containing derivatives of 1,3,4-thiadiazole and 1,2,4-triazole. Il Farmaco 1998, 53, 320–326. [Google Scholar]
- Küçükgüzel, S.G.; Rollas, S.; Erdeniz, H.; Kiraz, M. Synthesis, characterization and antimicrobial evaluation of ethyl 2-arylhydrazono-3-oxobutyrates. Eur. J. Med. Chem. 1999, 34, 153–160. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of “Antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Method. 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar]
- Delley, B. From molecules to solids with the dmol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar]
- Liu, Z.-Q.; Wu, D. How many peroxyl radicals can be scavenged by hydroxyl-substituted schiff bases in the oxidation of linoleic acid? J. Phys. Org. Chem. 2009, 22, 308–312. [Google Scholar]
- Laarhoven, L.J.J.; Mulder, P.; Wayner, D.D.M. Determination of bond dissociation enthalpies in solution by photoacoustic calorimetry. Accunts Chem. Res. 1999, 32, 342–349. [Google Scholar] [CrossRef]
- Crysalispro; Agilent Technologies Inc.: SantaClara, CA, USA, 2013.
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. A 2007, 64, 112–122. [Google Scholar] [CrossRef]
- Barbour, L.J. X-seed—A software tool for supramolecular crystallography. J. Supramol. Chem. 2001, 1, 189–191. [Google Scholar] [CrossRef]
- Gerhäuser, C.; Klimo, K.; Heiss, E.; Neumann, I.; Gamal-Eldeen, A.; Knauft, J.; Liu, G.-Y.; Sitthimonchai, S.; Frank, N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat. Res. 2003, 523, 163–172. [Google Scholar]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 03; Gaussian Inc.: Wallington, CT, USA, 2003. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry—The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2–11 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nazarbahjat, N.; Nordin, N.; Abdullah, Z.; Abdulla, M.A.; Yehye, W.A.; Halim, S.N.A.; Kee, C.H.; Ariffin, A. New Thiosemicarbazides and 1,2,4-Triazolethiones Derived from 2-(Ethylsulfanyl) Benzohydrazide as Potent Antioxidants. Molecules 2014, 19, 11520-11537. https://doi.org/10.3390/molecules190811520
Nazarbahjat N, Nordin N, Abdullah Z, Abdulla MA, Yehye WA, Halim SNA, Kee CH, Ariffin A. New Thiosemicarbazides and 1,2,4-Triazolethiones Derived from 2-(Ethylsulfanyl) Benzohydrazide as Potent Antioxidants. Molecules. 2014; 19(8):11520-11537. https://doi.org/10.3390/molecules190811520
Chicago/Turabian StyleNazarbahjat, Nafal, Nurdiana Nordin, Zanariah Abdullah, Mahmood Ameen Abdulla, Wageeh A. Yehye, Siti Nadiah Abdul Halim, Chin Hui Kee, and Azhar Ariffin. 2014. "New Thiosemicarbazides and 1,2,4-Triazolethiones Derived from 2-(Ethylsulfanyl) Benzohydrazide as Potent Antioxidants" Molecules 19, no. 8: 11520-11537. https://doi.org/10.3390/molecules190811520
APA StyleNazarbahjat, N., Nordin, N., Abdullah, Z., Abdulla, M. A., Yehye, W. A., Halim, S. N. A., Kee, C. H., & Ariffin, A. (2014). New Thiosemicarbazides and 1,2,4-Triazolethiones Derived from 2-(Ethylsulfanyl) Benzohydrazide as Potent Antioxidants. Molecules, 19(8), 11520-11537. https://doi.org/10.3390/molecules190811520