Synthesis, Immobilization and Catalytic Activity of a Copper(II) Complex with a Chiral Bis(oxazoline)
Abstract
:1. Introduction

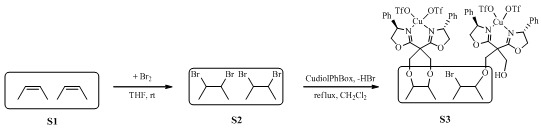
2. Results and Discussion

2.1. Characterization of the Materials
| Sample | %C a | %N a | %H a | %S a | %Cu b |
|---|---|---|---|---|---|
| S1 | 90.90 | 0.41 | 1.11 | ||
| S2 | 80.05 | 0.32 | 1.24 | ||
| S3 | 84.02 | 0.36 | 1.12 | 0.46 | 0.53 |
| Sample | %C | %O | %Br | %N | %F | %S | %Cu |
|---|---|---|---|---|---|---|---|
| S1 | 87.65 | 12.35 | |||||
| S2 | 85.62 | 13.78 | 0.60 | ||||
| S3 | 88.39 | 9.98 | 0.32 | 0.25 | 0.81 | 0.19 | 0.05 |
| Sample | ABET (m2/g)a | Amicro (m2/g) a | Aexternal (m2/g) a | VBJHads (cm3/g) | DBJHads (nm) b |
|---|---|---|---|---|---|
| S1 | 508 | 296 | 212 | 0.654 | 10.7 |
| S2 | 266 | 86 | 179 | 0.571 | 11.1 |
| S3 | 203 | 38 | 165 | 0.508 | 12.1 |


2.2. Catalytic Experiments

| Catalyst | Cycle | mol b (%) | %yield c | % ee d | S e | TON f | |
|---|---|---|---|---|---|---|---|
| Cu | diolPhBox | ||||||
| [Cu(OTf)2] | 1st | 0.7 | 33 | 0 | 50 | ||
| [Cu(OTf)2] + PhBox | 1st | 1.0 | 1.0 | 46 | 84 | 27 | 46 |
| [Cu(OTf)2] + diolPhBox | 1st | 1.1 | 1.1 | 47 | 84 | 26 | 43 |
| S3 | 1st | 0.7 | 0.1 | 38 | 38 | 3 | 55 |
| 2nd | 0.7 | 0.1 | 20 | 9 | 1 | 30 | |
3. Experimental Section
3.1. General Information
3.2. Synthesis of the Diolphbox
3.3. Bromination of the Starbon® 700
3.4. Immobilization of the Cu(II) Complex with Diolphbox Onto the Brominated Starbon® 700
3.5. Catalysis Experiments
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desimoni, G.; Faita, G.; Jorgensen, K.A. Update 1 of: C2-symmetric chiral bis(oxazoline) Ligands in asymmetric catalysis. Chem. Rev. 2011, 111, 284–437. [Google Scholar]
- Matsumura, Y.; Maki, T.; Murakami, S.; Onomura, O. Copper ion-induced activation and asymmetric benzoylation of 1,2-diols: Kinetic chiral molecular recognition. J. Am. Chem. Soc. 2003, 125, 2052–2053. [Google Scholar]
- Rechavi, D.; Lemaire, M. Enantioselective catalysis using heterogeneous bis(oxazoline) ligands: Which factors influence the enantioselectivity? Chem. Rev. 2002, 102, 3467–3493. [Google Scholar] [CrossRef]
- Trindade, A.F.; Gois, P.M.P.; Afonso, C.A.M. Recylable stereoselective catalysts. Chem. Rev. 2009, 109, 418–513. [Google Scholar]
- Fraile, J.M.; Garcia, J.I.; Mayoral, J.A. Noncovalent immobilization of enantioselective catalysts. Chem. Rev. 2009, 109, 360–417. [Google Scholar]
- Fraile, J.M.; Garcia, J.I.; Herrerias, C.I.; Mayoral, J.A.; Pires, E. Enantioselective catalysis with chiral complexes immobilized on nanostructured supports. Chem. Soc. Rev. 2009, 38, 695–706. [Google Scholar]
- Silva, A.R.; Albuquerque, H.; Borges, S.; Siegel, R.; Mafra, L.; Carvalho, A.P.; Pires, J. Strategies for copper bis(oxazoline) immobilization onto porous silica based materials. Micropor. Mesopor. Mater. 2012, 158, 26–38. [Google Scholar]
- Silva, A.R.; Guimarães, V.; Carvalho, A.P.; Pires, J. The role of the support properties in the catalytic performance of an anchored copper(II) aza-bis(oxazoline) in mesoporous silicas and their carbon replicas. Catal. Sci. Technol. 2013, 3, 659–672. [Google Scholar]
- Silva, A.R.; Carneiro, L.; Carvalho, A.P.; Pires, J. Asymmetric benzoylation of hydrobenzoin by copper(II) bis(oxazoline) anchored onto ordered mesoporous silicas and their carbon replicas. Catal. Sci. Technol. 2013, 3, 2415–2424. [Google Scholar]
- Albuquerque, H.; Carneiro, L.; Carvalho, A.P.; Pires, J.; Silva, A.R. Enantioselective cyclopropanation and aziridination of styrene catalyzed by copper(II) bis(oxazoline) anchored onto mesoporous materials. Polyhedron 2014, 79, 315–323. [Google Scholar]
- Lee, S.S.; Hadinoto, S.; Ying, J.Y. Improved enantioselectivity of immobilized chiral bisoxazolines by partial precapping of the siliceous mesocellular foam support with trimethylsilyl groups. Adv. Synth. Catal. 2006, 348, 1248–1254. [Google Scholar]
- Lim, J.; Riduan, S.N.; Lee, S.S.; Ying, J.Y. Siliceous mesocellular foam-supported aza(bisoxazoline)-copper catalysts. Adv. Synth. Catal. 2008, 350, 1295–1308. [Google Scholar]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar]
- Silva, A.R.; Figueiredo, J.L.; Freire, C.; de Castro, B. Manganese(III) salen complexes anchored onto activated carbon as heterogeneous catalysts for the epoxidation of olefins. Micropor. Mesopor. Mat. 2004, 68, 83–89. [Google Scholar]
- Silva, A.R.; Budarin, V.; Clark, J.H.; de Castro, B.; Freire, C. Chiral manganese(III) Schiff base complexes anchored onto activated carbon as enantioselective heterogeneous catalysts for alkene epoxidation. Carbon 2005, 43, 2096–2105. [Google Scholar]
- Silva, A.R.; Budarin, V.; Clark, J.H.; Freire, C.; de Castro, B. Organo-functionalized activated carbons as supports for the covalent attachment of a chiral manganese(III) salen complex. Carbon 2007, 45, 1951–1964. [Google Scholar]
- Silva, A.R.; Budarin, V.; Clark, J.H. Microwave-Assisted immobilization of manganese salen complexes: Increased activity and chemoselectivity in catalytic epoxidation. Chem. Cat. Chem. 2013, 5, 895–898. [Google Scholar]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar]
- Budarin, V.; Clark, J.H.; Hardy, J.J.E.; Luque, R.; Milkowski, K.; Tavener, S.J.; Wilson, A.J. Starbons: New starch-derived mesoporous carbonaceous materials with tunable properties. Angew. Chem. Int. Ed. 2006, 45, 3782–3786. [Google Scholar]
- Rechavi, D.; Lemaire, M. Heterogenization of a chiral bis(oxazoline) catalyst by grafting onto silica. Org. Lett. 2001, 3, 2493–2496. [Google Scholar]
- Budarin, V.L.; Clark, J.H.; Tavener, S.J.; Wilson, K. Chemical reactions of double bonds in activated carbon: Microwave and bromination methods. Chem. Commun. 2004, 2736–2737. [Google Scholar]
- Colomer, J.-F.; Marega, R.; Traboulsi, H.; Meneghetti, M.; van Tendeloo, G.; Bonifazi, D. Microwave-assisted bromination of double-wall carbon nanotubes. Chem. Mater. 2009, 21, 4747–4749. [Google Scholar]
- Silva, A.R.; Albuquerque, H.; Fontes, A.; Borges, S.; Martins, A.; Carvalho, A.P.; Pires, J. Copper bis(oxazoline) encapsulated in zeolites and its application as heterogeneous catalysts for the cyclopropanation of styrene. Ind. Eng. Chem. Res. 2011, 50, 11495–11501. [Google Scholar]
- Silva, A.R.; Guimarães, V.; Carneiro, L.; Martins, A.; Carvalho, A.P.; Pires, J. Copper(II) aza-bis(oxazoline) complex anchored and encapsulated into MCM-22 derived materials as heterogeneous catalysts for the transformation of alkenes. Micropor. Mesopor. Mat. 2013, 179, 231–241. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Carneiro, L.; Silva, A.R.; Shuttleworth, P.S.; Budarin, V.; Clark, J.H. Synthesis, Immobilization and Catalytic Activity of a Copper(II) Complex with a Chiral Bis(oxazoline). Molecules 2014, 19, 11988-11998. https://doi.org/10.3390/molecules190811988
Carneiro L, Silva AR, Shuttleworth PS, Budarin V, Clark JH. Synthesis, Immobilization and Catalytic Activity of a Copper(II) Complex with a Chiral Bis(oxazoline). Molecules. 2014; 19(8):11988-11998. https://doi.org/10.3390/molecules190811988
Chicago/Turabian StyleCarneiro, Liliana, Ana R. Silva, Peter S. Shuttleworth, Vitaly Budarin, and James H. Clark. 2014. "Synthesis, Immobilization and Catalytic Activity of a Copper(II) Complex with a Chiral Bis(oxazoline)" Molecules 19, no. 8: 11988-11998. https://doi.org/10.3390/molecules190811988
APA StyleCarneiro, L., Silva, A. R., Shuttleworth, P. S., Budarin, V., & Clark, J. H. (2014). Synthesis, Immobilization and Catalytic Activity of a Copper(II) Complex with a Chiral Bis(oxazoline). Molecules, 19(8), 11988-11998. https://doi.org/10.3390/molecules190811988