Caffeic Acid Inhibits the Formation of 7-Carboxyheptyl Radicals from Oleic Acid under Flavin Mononucleotide Photosensitization by Scavenging Singlet Oxygen and Quenching the Excited State of Flavin Mononucleotide
Abstract
:1. Introduction
2. Results and Discussion
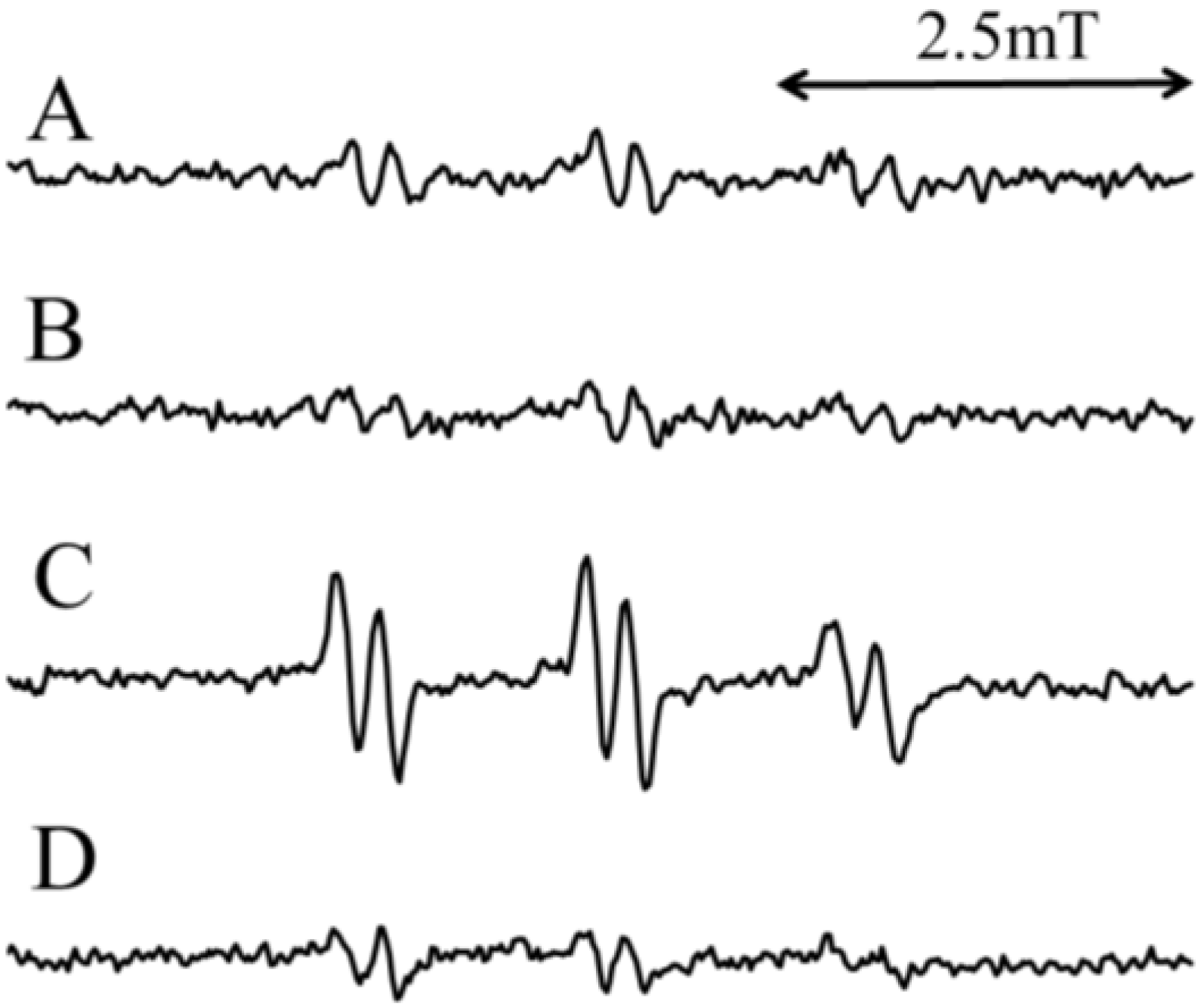
2.1. Electron Spin Resonance (ESR) Analysis of the Standard Reaction Mixture (I) with CA
2.2. High Performance Liquid Chromatography (HPLC)-ESR Analyses of the Standard D2O Reaction Mixture (I) with CA
2.3. Effect of CA and Its Related Compounds on the Formation of Free Radicals



2.4. Iron Chelation by CA

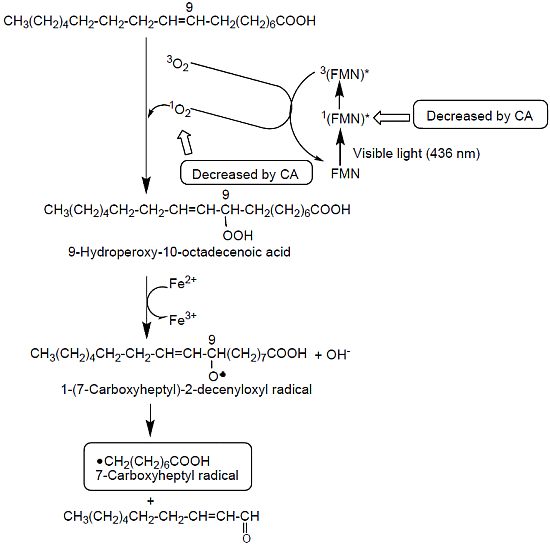
2.5. A Possible Reaction Path for the Formation of 7-Carboxyheptyl Radicals in the Standard D2O Reaction Mixture (I)


2.6. Effects of CA on the Singlet Excited State of FMN and 1O2


3. Experimental Section
3.1. Chemicals
3.2. Standard Reaction Mixture (I)
3.3. Standard Reaction Mixture (II) with CA
3.4. Standard Reaction Mixture (III)
3.5. ESR Analyses
3.6. HPLC-ESR Analyses
3.7. Visible Absorption Analyses
3.8. Fluorescence Measurements
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef]
- Barua, R.S.; Ambrose, J.A. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1460–1467. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Forchhammer, L.; Møller, P.; Simonsen, J.; Glasius, M.; Wåhlin, P.; Raaschou-Nielsen, O.; Loft, S. Exposure to ultrafine particles from ambient air and oxidative stress-induced DNA damage. Environ. Health Perspect. 2007, 115, 1177–1182. [Google Scholar] [CrossRef]
- Demetriou, C.A.; Raaschou-Nielsen, O.; Loft, S.; Møller, P.; Vermeulen, R.; Palli, D.; Chadeau-Hyam, M.; Xun, W.W.; Vineis, P. Biomarkers of ambient air pollution and lung cancer: A systematic review. Occup. Environ. Med. 2012, 69, 619–627. [Google Scholar] [CrossRef]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef]
- Umeno, A.; Shichiri, M.; Ishida, N.; Hashimoto, Y.; Abe, K.; Kataoka, M.; Yoshino, K.; Hagihara, Y.; Aki, N.; Funaki, M.; et al. Singlet oxygen induced products of linoleates, 10- and 12-(Z,E)-hydroxyoctadecadienoic acids (HODE), can be potential biomarkers for early detection of type 2 diabetes. PLoS One 2013, 8, e63542. [Google Scholar] [CrossRef]
- Husain, S.R.; Josiane, C.; Pierre, C. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar]
- Sanz, M.J.; Ferrandiz, M.L.; Cejudo, M.; Terencio, M.C.; Gil, B.; Bustos, G.; Ubeda, A.; Gunasegaran, R.; Alcaraz, M.J. Influence of a series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotica 1994, 24, 689–699. [Google Scholar] [CrossRef]
- Afanas’ev, I.B.; Dorozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 1989, 38, 1763–1769. [Google Scholar] [CrossRef]
- Morel, I.; Lescoat, G.; Cogrel, P.; Sergent, O.; Pasdeloup, N.; Brissot, P.; Cillard, P.; Cillard, J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Van Acker, S.A.; van den Berg, D.J.; Tromp, M.N.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 4, 101–123. [Google Scholar] [CrossRef]
- Salazar-Martinez, E.; Willett, W.C.; Ascherio, A.; Manson, J.E.; Leitzmann, M.F.; Stampfer, M.J.; Hu, F.B. Coffee consumption and risk for type 2 diabetes mellitus. Ann. Intern. Med. 2004, 140, 1–8. [Google Scholar] [CrossRef]
- Van Dam, R.M.; Hu, F.B. Coffee consumption and risk of type 2 diabetes: A systematic review. JAMA 2005, 294, 97–104. [Google Scholar] [CrossRef]
- Andersen, L.F.; Jacobs, D.R., Jr.; Carlsen, M.H.; Blomhoff, R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women’s Health Study. Am. J. Clin. Nutr. 2006, 83, 1039–1046. [Google Scholar]
- Butt, M.S.; Sultan, M.T. Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2011, 51, 363–373. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates-nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Sakakibara, H.; Honda, Y.; Nakagawa, S.; Ashida, H.; Kanazawa, K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003, 51, 571–581. [Google Scholar] [CrossRef]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic acid and ferulic acid inhibit UVA-induced matrix metalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar]
- Lekse, J.M.; Xia, L.; Stark, J.; Morrow, J.D.; May, J.M. Plant catechols prevent lipid peroxidation in human plasma and erythrocytes. Mol. Cell. Biochem. 2001, 226, 89–95. [Google Scholar] [CrossRef]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim. Biophys. Acta 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Ito, H.; Miyazaki, T.; Ono, M.; Sakurai, H. Antiallergic activities of rabdosiin and its related compounds: Chemical and biochemical evaluations. Bioorg. Med. Chem. 1998, 6, 1051–1056. [Google Scholar] [CrossRef]
- Yamada, Y.; Yasui, H.; Sakurai, H. Suppressive effect of caffeic acid and its derivatives on the generation of UVA-induced reactive oxygen species in the skin of hairless mice and pharmacokinetic analysis on organ distribution of caffeic acid in ddY mice. Photochem. Photobiol. 2006, 82, 1668–1676. [Google Scholar]
- Hsieh, C.L.; Yen, G.C.; Chen, H.Y. Antioxidant activities of phenolic acids on ultraviolet radiation-induced erythrocyte and low density lipoprotein oxidation. J. Agric. Food Chem. 2005, 53, 6151–6155. [Google Scholar] [CrossRef]
- Ikeda, H.; Kimura, Y.; Masaki, M.; Iwahashi, H. Caffeic acid inhibits the formation of 1-hydroxyethyl radical in the reaction mixture of rat liver microsomes with ethanol partly through its metal chelating activity. J. Clin. Biochem. Nutr. 2011, 48, 187–193. [Google Scholar]
- Iwahashi, H. Some polyphenols inhibit the formation of pentyl radical and octanoic acid radical in the reaction mixture of linoleic acid hydroperoxide with ferrous ions. Biochem. J. 2000, 346, 265–273. [Google Scholar] [CrossRef]
- Mori, H.; Iwahashi, H. Formation of 7-carboxyheptyl radical induced by singlet oxygen in the reaction mixture of oleic acid, riboflavin and ferrous ion under the UVA irradiation. J. Clin. Biochem. Nutr. 2011, 49, 141–146. [Google Scholar]
- Baier, J.; Maier, M.; Engel, E.; Landthaler, M.; Baumler, W. Singlet oxygen generation by UVA light exposure of endogenous photosensitizers. Biophys. J. 2006, 91, 1452–1459. [Google Scholar] [CrossRef]
- Mori, H.M.; Iwahashi, H. Detection and identification of 1-methylethyl and methyl radicals generated by irradiating tea tree (Melaleuca alternifolia) oil with visible light (436 nm) in the presence of flavin mononucleotide and ferrous ion. Free Radic. Res. 2013, 47, 657–663. [Google Scholar] [CrossRef]
- Miyamoto, S.; Ronsein, G.E.; Prado, F.M.; Uemi, M.; Corrêa, T.C.; Toma, I.N.; Bertolucci, A.; Oliveira, M.C.; Motta, F.D.; Medeiros, M.H.; et al. Biological hydroperoxides and singlet molecular oxygen generation. IUBMB Life 2007, 59, 322–331. [Google Scholar] [CrossRef]
- Mori, H.; Iwahashi, H. Identification of the radicals formed in the reactions of some endogenous photosensitizers with oleic acid under the UVA irradiation. J. Clin. Biochem. Nutr. 2012, 51, 170–177. [Google Scholar]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [CrossRef]
- Lion, Y.; Delmelle, M.; van de Vorst, A. New method of detecting singlet oxygen production. Nature 1976, 263, 442–443. [Google Scholar] [CrossRef]
- Nakamura, K.; Ishiyama, K.; Ikai, H.; Kanno, T.; Sasaki, K.; Niwano, Y.; Kohno, M. Reevaluation of analytical methods for photogenerated singlet oxygen. J. Clin. Biochem. Nutr. 2011, 49, 87–95. [Google Scholar] [CrossRef]
- Wilkinson, F.; Brummer, J.G. Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. J. Phys. Chem. Ref. Data 1981, 10, 809–999. [Google Scholar] [CrossRef]
- Scurlock, R.; Rougee, M.; Bensasson, R.V. Redox properties of phenols, their relationships to singlet oxygen quenching and to their inhibitory effects on benzo(a)pyrene-induced neoplasia. Free Radic. Res. Commun. 1990, 8, 251–258. [Google Scholar] [CrossRef]
- Tournaire, C.; Croux, S.; Maurette, M.T.; Beck, I.; Hocquaux, M.; Braun, A.M.; Oliveros, E. Antioxidant activity of flavonoids: Efficiency of singlet oxygen (1 delta g) quenching. J. Photochem. Photobiol. B 1993, 19, 205–215. [Google Scholar] [CrossRef]
- Miskoski, S.; Soltermann, A.T.; Molina, P.G.; Günther, G.; Zanocco, A.L.; García, N.A. Sensitized photooxidation of thyroidal hormones. Evidence for heavy atom effect on singlet molecular oxygen [O2(1Δg)]-mediated photoreactions. Photochem. Photobiol. 2005, 81, 325–332. [Google Scholar] [CrossRef]
- Mukai, K.; Ouchi, A.; Takahashi, S.; Aizawa, K.; Inakuma, T.; Terao, J.; Nagaoka, S. Development of singlet oxygen absorption capacity (SOAC) assay method. 3. Measurements of the SOAC values for phenolic antioxidants. J. Agric. Food Chem. 2012, 60, 7905–7916. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asano, M.; Iwahashi, H. Caffeic Acid Inhibits the Formation of 7-Carboxyheptyl Radicals from Oleic Acid under Flavin Mononucleotide Photosensitization by Scavenging Singlet Oxygen and Quenching the Excited State of Flavin Mononucleotide. Molecules 2014, 19, 12486-12499. https://doi.org/10.3390/molecules190812486
Asano M, Iwahashi H. Caffeic Acid Inhibits the Formation of 7-Carboxyheptyl Radicals from Oleic Acid under Flavin Mononucleotide Photosensitization by Scavenging Singlet Oxygen and Quenching the Excited State of Flavin Mononucleotide. Molecules. 2014; 19(8):12486-12499. https://doi.org/10.3390/molecules190812486
Chicago/Turabian StyleAsano, Marie, and Hideo Iwahashi. 2014. "Caffeic Acid Inhibits the Formation of 7-Carboxyheptyl Radicals from Oleic Acid under Flavin Mononucleotide Photosensitization by Scavenging Singlet Oxygen and Quenching the Excited State of Flavin Mononucleotide" Molecules 19, no. 8: 12486-12499. https://doi.org/10.3390/molecules190812486
APA StyleAsano, M., & Iwahashi, H. (2014). Caffeic Acid Inhibits the Formation of 7-Carboxyheptyl Radicals from Oleic Acid under Flavin Mononucleotide Photosensitization by Scavenging Singlet Oxygen and Quenching the Excited State of Flavin Mononucleotide. Molecules, 19(8), 12486-12499. https://doi.org/10.3390/molecules190812486