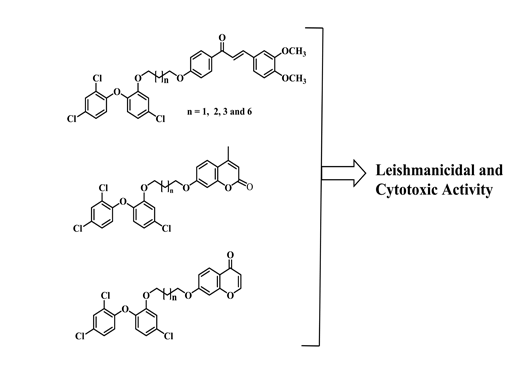
Synthesis, Leishmanicidal and Cytotoxic Activity of Triclosan-Chalcone, Triclosan-Chromone and Triclosan-Coumarin Hybrids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry


2.2. Antileishmanial and Cytotoxic Activities
| Compound | Cytotoxicity | Leishmanicidal activity | SI d | |
|---|---|---|---|---|
| LC50 (μg/mL, µM) a | % Inhibition b | EC50 (μg/mL, µM) c | ||
| 7 | >200.0, >326.7 | 67.4 ± 17.2 | 9.4 ± 1.3, 15.4 | >21.3 |
| 8 | >200.0, >319.4 | 67.8 ± 19.0 | 10.2 ± 1.8, 16.3 | >19.6 |
| 9 | >200.0, >311,4 | 69.5 ± 8.6 | 13.5 ± 3.6, 21.1 | >14.8 |
| 10 | >200.0, >293.2 | 23.3 ± 5.2 | NE e | NC f |
| 16 | >200.0, >396.8 | 34.0 ± 0.3 | NE e | NC f |
| 17 | >200.0, >386.1 | 57.1 ± 11.5 | 27.5 ± 0.8, 53.1 | >7.3 |
| 18 | >200.0, >375.9 | 0.0 | NE e | NC f |
| 19 | >200.0, >348.4 | 0.0 | NE e | NC f |
| 25 | 6.4 ± 0.8, 13.1 | 94.4 ± 2.9 | 2.7 ± 0.4, 5.5 | 2.4 |
| 26 | 15.8 ± 4.3, 31.3 | 91.0 ± 9.6 | 7.5 ± 0.2, 14.9 | 2.1 |
| 27 | 25.8 ± 4.2, 49.8 | 75.5 ± 1.5 | 16.0 ± 1.0, 30.9 | 1.6 |
| 28 | 80.0 ± 18.5, 142.8 | 28.4 ± 2.1 | NE e | NC f |
| Triclosan | 22.1 ± 3.1, 76.3 | 61.8 ± 5.5 | 18.3 ± 2.01, 63.2 | 1.3 |
| 3,4-Dimethoxy-4'-hydroxychalcone ( 6) | 13.9 ± 1.4, 48.9 | 52.4 ± 6.5 | 20.03 ± 1.4, 70.5 | 0.7 |
| 7-Hydroxychromone ( 20) | >200.0, >1234.6 | 14.8 ± 0.9 | NE e | NC f |
| 7-Hydroxy-4-methylcoumarin ( 11) | 98.2 ± 6.7, 557.4 | 15.3 ± 0.1 | NE e | NC f |
| Sb(V) g | 495.9 + 55.6 | 79.4 ± 2.1 h | 6.3 + 0.9 | 78.7 |
| Amphotericin B | 42.1 ± 2.0, 45.6 | 69.1 ± 1.3 i | 0.06 ± 0.01, 0.1 | 592 |
3. Experimental Section
3.1. Chemical Synthesis
3.1.1. General Remarks
3.1.2. General Procedure for the Synthesis of Bromoalkyl Derivatives
3.1.3. General Procedure for the Synthesis of Triclosan-Chalcone Hybrids
3.1.4. General Procedure for the Synthesis of Triclosan-Coumarin and Triclosan-Chromone Hybrids
3.2. Biological Activity Assays
3.2.1. In Vitro Cytotoxic Activity in Mammalian Cells
3.2.2. Activity against Intracellular Amastigotes
3.2.3. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Den Boer, M. WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One 2012, 7, e35671. [Google Scholar]
- World Health Organization. Control of Leishmaniasis: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases. In Presented at WHO Technical Report Series, No. 949, Geneva, Switzerland, 22–26 March 2010.
- Fontenele e Silva, J.S.; Galvao, T.F.; Pereira, M.G.; Silva, M.T. Treatment of american tegumentary leishmaniasis in special populations: A summary of evidence. Rev. Soc. Bras. Med. Trop. 2013, 46, 669–677. [Google Scholar] [CrossRef]
- Tanne, J.H. How collaboration is providing new drugs for neglected diseases. BMJ 2012, 344, e2453. [Google Scholar] [CrossRef]
- Kapoor, M.; Reddy, C.; Krishnasastry, M.V.; Surolia, N.; Surolia, A. Slow-tight-binding inhibition of enoyl-acyl carrier protein reductase from Plasmodium falciparum by triclosan. Biochem. J. 2004, 381, 719–724. [Google Scholar] [CrossRef]
- Perozzo, R.; Kuo, M.; Sidhu, A.; Valiyaveettil, J.T.; Bittman, R.; Jacobs, W.R., Jr.; Fidock, D.A.; Sacchettini, J.C. Structural elucidation of the specificity of the antibacterial agent triclosan for malarial enoyl acyl carrier protein reductase. J. Biol. Chem. 2002, 277, 13106–13114. [Google Scholar] [CrossRef]
- Surolia, N.; Surolia, A. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 2001, 7, 167–173. [Google Scholar] [CrossRef]
- McLeod, R.; Muench, S.P.; Rafferty, J.B.; Kyle, D.E.; Mui, E.J.; Kirisits, M.J.; Mack, D.G.; Roberts, C.W.; Samuel, B.U.; Lyons, R.E.; et al. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of apicomplexan Fab I. Int. J. Parasitol. 2001, 31, 109–113. [Google Scholar] [CrossRef]
- Arango, V.; Domínguez, J.J.; Cardona, W.; Robledo, S.M.; Muñoz, D.L.; Figadere, B.; Saéz, J. Synthesis and leishmanicidal activity of quinoline-triclosan and quinoline-eugenol hybrids. Med. Chem. Res. 2012, 21, 3445–3454. [Google Scholar] [CrossRef]
- Kayser, O.; Kiderlen, A.F. In vitro leishmanicidal activity of naturally occurring chalcones. Phytother. Res. 2001, 15, 148–152. [Google Scholar] [CrossRef]
- Liu, M.; Wilairat, P.; Croft, S.L.; Tand, A.L.; Go, M.L. Structure-activity relationships of antileishmanial and antimalarial chalcones. Bioorg. Med. Chem. 2003, 11, 2729–2738. [Google Scholar] [CrossRef]
- Boeck, P.; Bandeira Falcão, C.A.; Leal, P.C.; Yones, R.A.; Filho, V.C.; Torres-Santos, E.C.; Rossi-Bergmann, B. Synthesis of chalcone analogues with increased antileishmanial activity. Bioorg. Med. Chem. 2006, 14, 1538–1545. [Google Scholar] [CrossRef]
- Chen, M.; Zhai, L.; Christensen, S.B.; Theander, T.G.; Kharazmi, A. Inhibition of Fumarate Reductase in Leishmania major and L. donovani by Chalcones. Antimicrobiol. Agents Chemother. 2001, 45, 2023–2029. [Google Scholar] [CrossRef]
- Morabito, G.; Trombetta, D.; Singh Brajendra, K.; Prasad Ashok, K.; Parmar Virinder, S.; Naccari, C.; Saija, A.; Cristani, M.; Firuzi, O.; Saso, L. Antioxidant properties of 4-methylcoumarins in in vitro cell-free systems. Biochimie 2010, 92, 1107–1117. [Google Scholar]
- Grimm, E.L.; Brideau, C.; Chauret, N.; Chan, C.C.; Delorme, D.; Ducharme, Y.; Ethier, D.; Falgueyret, J.P.; Friesen, R.W; Guay, J.; et al. Substituted coumarins as potent 5-lipoxygenase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 2528–2531. [Google Scholar] [CrossRef]
- Montagner, C.; de Souza, S.M.; Groposoa, C.; Delle Monache, F.; Smânia, E.F.; Smânia, A., Jr. Antifungal activity of coumarins. Z. Naturforsch. 2008, 63c, 21–28. [Google Scholar]
- Paya, M.; Goodwin, P.A.; De Las Heras, B.; Hoult, R.S. Superoxide scavenging activity in leukocytesand absence of cellular toxicity of a series of coumarins. Biochem. Pharmacol. 1994, 48, 445–451. [Google Scholar] [CrossRef]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef]
- Horton, D.A.; Boume, G.T.; Smythe, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef]
- Hadjeri, M.; Barbier, M.; Ronot, X.; Mariotte, A.M.; Boumendjel, A.; Boutonnat, J. Modulation of P-glycoprotein mediated multidrug resistance by flavonoid derivatives and analogues. J. Med. Chem. 2003, 46(11), 2125–2131. [Google Scholar]
- Ellis, G.P.; Barker, G. Chromone-2-and -3-carboxylic acids and their derivatives. Prog. Med. Chem. 1972, 9, 65–116. [Google Scholar] [CrossRef]
- Houghton, P.J. Chemistry and biological activity of natural and semi-synthetic chromone alkaloids. Stud. Nat. Prod. Chem. 2000, 21, 123–155. [Google Scholar] [CrossRef]
- Mallick, S.; Dutta, A.; Ghosh, J.; Maiti, S.; Mandal, A.K.; Banerjee, R.; Bandyopadhyay, C.; Pal, C. Protective therapy with novel chromone derivative against Leishmania donovani infection induces Th1 response in vivo. Chemotherapy 2011, 57, 388–393. [Google Scholar] [CrossRef]
- Baloch, N.; Alkahraman, Y.; Zaidi, M.; Madkour, H. Evaluation of 6, 8-Dichloro-2-methyl-4H-chromen-4-one derivatives as antileishmanial agents. Glob. J. Sci. Front. Res. Chem. 2012, 12, 26–32. [Google Scholar]
- Napolitano, H.B.; Silva, M.; Ellena, J.; Rodrigues, B.D.; Almeida, A.L.; Vieira, P.C.; Oliva, G.; Thiemann, O.H. Aurapten, a coumarin with growth inhibition against Leishmania major promastigotes. Braz. J. Med. Biol. Res. 2004, 37, 1847–1852. [Google Scholar] [CrossRef]
- Arango, V.; Robledo, S.; Séon-Méniel, B.; Figadère, B.; Cardona, W.; Saez, J.; Otalvaro, F. Coumarins from Galipea panamensis and their activity against Leishmania panamensis. J. Nat. Prod. 2010, 73, 1012–1014. [Google Scholar] [CrossRef]
- Pierson, J.T.; Dumètre, A.; Hutter, S.; Delmas, F.; Laget, M.; Finet, J.P.; Azas, N.; Combes, S. Synthesis and antiprotozoal activity of 4-arylcoumarins. Eur. J. Med. Chem. 2010, 45, 864–869. [Google Scholar] [CrossRef]
- Keith, C.T.; Borisy, A.A; Stockwell, B.R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 2005, 4, 71–78. [Google Scholar] [CrossRef]
- Roth, B.L.; Sheffler, D.J.; Kroeze, W.K. Magic shotguns versus magic bullets: Selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 2004, 3, 353–359. [Google Scholar] [CrossRef]
- Musonda, C.C.; Whitlock, G.A.; Witty, M.J.; Brun, R.; Kaiser, M. Chloroquine-astemizole hybridswith potent in vitro and in vivo antiplasmodial activity. Bioorg. Med. Chem. Lett. 2009, 19, 481–484. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- Bollini, M.; Casal, J.J.; Bruno, A.M. Design, synthesis, and antitumor activity of new bis-aminomethylnaphthalenes. Bioorg. Med. Chem. 2008, 16, 8003–8010. [Google Scholar] [CrossRef]
- Opsenica, I.; Opsenica, D.; Lanteri, C.A.; Anova, L.; Milhous, W.K.; Smith, K.S.; Solaja, B.A. New chimeric antimalarials with 4-aminoquinoline moiety linked to a tetraoxane skeleton. J. Med. Chem. 2008, 51, 6216–6219. [Google Scholar] [CrossRef]
- Walsh, J.J.; Coughlan, D.; Heneghan, N.; Gaynor, C.; Bell, A. A novel artemisinin-quinine hybrid with potent antimalarial activity. Bioorg. Med. Chem. Lett. 2007, 17, 3599–3602. [Google Scholar] [CrossRef]
- Peng, Y.; Song, G. Combined microwave and ultrasound assisted Williamson ether synthesis in the absence of phase-transfer catalysts. Green Chem. 2002, 4, 349–351. [Google Scholar] [CrossRef]
- Peyman, S.; Minoo, D.; Mohammad, A.Z.; Mohammad, A.B. Silica sulfuric acid as an efficient and reusable reagent for crossed-aldol condensation of ketones with aromatic aldehydes under solvent-free conditions. J. Braz. Chem. Soc. 2004, 15, 773–776. [Google Scholar] [CrossRef]
- Manhas, M.S.; Ganguly, S.N.; Mukherjee, S.; Jain, A.K.; Bose, A.K. Microwave initiated reactions: Pechmann coumarin synthesis, Biginelli reaction, and acylation. Tetrahedron Lett. 2006, 47, 2423–2425. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, X.B.; Xie, S.S.; Jian, N.; Wang, K.D.; Yao, H.Q.; Sun, H.B.; Kong, L.Y. Multifunctional tacrine-flavonoid hybrids with cholinergic, β-amyloid-reducing, and metal chelatingproperties for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2013, 69, 632–646. [Google Scholar] [CrossRef]
- Xie, S.S.; Wang, X.B.; Li, J.Y.; Yang, L.; Kong, L.Y. Design, synthesis and evaluation of novel tacrine-coumarin hybrids as multifunctional cholinesterase inhibitors against Alzheimer’s disease. Eur. J. Med. Chem. 2013, 64, 540–553. [Google Scholar] [CrossRef]
- Taylor, V.M.; Cedeño, D.L.; Muñoz, D.L.; Jones, M.A.; Lash, T.D.; Young, A.M.; Constantino, M.H.; Esposito, N.; Velez, I.D.; Robledo, S.M. In vitro and in vivo studies of the utility of dimethyl and diethyl carbaporphyrin ketals in treatment of cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2011, 55, 4755–4764. [Google Scholar] [CrossRef]
- Pulido, S.A.; Muñoz, D.L.; Restrepo, A.M.; Mesa, C.V.; Alzate, J.F.; Vélez, I.D.; Robledo, S.M. Improvement of the green fluorescent protein reporter system in Leishmania spp. for the in vitro and in vivo screening of antileishmanial drugs. Acta Trop. 2012, 122, 36–45. [Google Scholar] [CrossRef]
- Cardona, G.W.; Sáez, V.J. Antiprotozoal activity of α,β-unsaturated d-lactones: Promising compounds for the development of new therapeutic alternatives. Trop. J. Pharm. Res. 2011, 10, 671–680. [Google Scholar]
- Cardona, W.; Guerra, D.; Restrepo, A. Reactivity of δ-substituted α,β-unsaturated cyclic lactones with antileishmanial activity. Mol. Simul. 2014, 40, 477–484. [Google Scholar] [CrossRef]
- Otero, E.; Robledo, S.M.; Díaz, S.; Carda, M.; Muñoz, D.; Paños, J.; Vélez, I.D.; Cardona, W. Synthesis and leishmanicidal activity of cinnamic acid esters: Structure-activity relationship. Med. Chem. Res. 2014, 23, 1378–1386. [Google Scholar] [CrossRef]
- Finney, J.D. Probit Analysis: Statistical Treatment of the Sigmoid Response Curve, 3rd ed.; Cambridge University Press: Cambridge, UK, 1978; p. 550. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Otero, E.; Vergara, S.; Robledo, S.M.; Cardona, W.; Carda, M.; Vélez, I.D.; Rojas, C.; Otálvaro, F. Synthesis, Leishmanicidal and Cytotoxic Activity of Triclosan-Chalcone, Triclosan-Chromone and Triclosan-Coumarin Hybrids. Molecules 2014, 19, 13251-13266. https://doi.org/10.3390/molecules190913251
Otero E, Vergara S, Robledo SM, Cardona W, Carda M, Vélez ID, Rojas C, Otálvaro F. Synthesis, Leishmanicidal and Cytotoxic Activity of Triclosan-Chalcone, Triclosan-Chromone and Triclosan-Coumarin Hybrids. Molecules. 2014; 19(9):13251-13266. https://doi.org/10.3390/molecules190913251
Chicago/Turabian StyleOtero, Elver, Sebastián Vergara, Sara M. Robledo, Wilson Cardona, Miguel Carda, Ivan D. Vélez, Carlos Rojas, and Felipe Otálvaro. 2014. "Synthesis, Leishmanicidal and Cytotoxic Activity of Triclosan-Chalcone, Triclosan-Chromone and Triclosan-Coumarin Hybrids" Molecules 19, no. 9: 13251-13266. https://doi.org/10.3390/molecules190913251
APA StyleOtero, E., Vergara, S., Robledo, S. M., Cardona, W., Carda, M., Vélez, I. D., Rojas, C., & Otálvaro, F. (2014). Synthesis, Leishmanicidal and Cytotoxic Activity of Triclosan-Chalcone, Triclosan-Chromone and Triclosan-Coumarin Hybrids. Molecules, 19(9), 13251-13266. https://doi.org/10.3390/molecules190913251