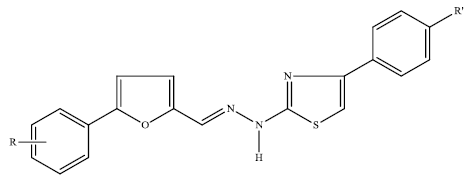
Synthesis and in Vitro Evaluation of New Nitro-Substituted Thiazolyl Hydrazone Derivatives as Anticandidal and Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion

| Compound | R | R' | Yield (%) | M.p. (°C) |
|---|---|---|---|---|
| 1 | p-NO2 | H | 80 | 213–214 |
| 2 | p-NO2 | NO2 | 85 | 252–254 |
| 3 | p-NO2 | F | 82 | 241–242 |
| 4 | p-NO2 | Cl | 81 | 229–231 |
| 5 | p-NO2 | Br | 80 | 243–244 |
| 6 | p-NO2 | CH3 | 90 | 236–237 |
| 7 | p-NO2 | OCH3 | 90 | 232–233 |
| 8 | p-Cl-o-NO2 | H | 75 | 194–195 |
| 9 | p-Cl-o-NO2 | NO2 | 95 | 216–220 |
| 10 | p-Cl-o-NO2 | F | 90 | 208–209 |
| 11 | p-Cl-o-NO2 | Cl | 88 | 186–187 |
| 12 | p-Cl-o-NO2 | Br | 90 | 199–201 |
| 13 | p-Cl-o-NO2 | CH3 | 78 | 166–167 |
| 14 | p-Cl-o-NO2 | OCH3 | 90 | 206–207 |
| Compound | Candida Species Tested | |||||
|---|---|---|---|---|---|---|
| C. albicans | C. utilis | C. tropicalis | C. krusei | C. parapsilosis | C. glabrata | |
| 1 | 500 | 500 | 1000 | 500 | 500 | 1000 |
| 2 | 500 | 500 | 500 | 500 | 500 | 1000 |
| 3 | 500 | 500 | 500 | 500 | 500 | 1000 |
| 4 | 500 | 500 | 500 | 500 | 1000 | 500 |
| 5 | 500 | 500 | 500 | 1000 | 500 | 1000 |
| 6 | 500 | 500 | 1000 | 1000 | 500 | 500 |
| 7 | 500 | 500 | 500 | 500 | 500 | 1000 |
| 8 | 500 | 500 | 500 | 500 | 1000 | 1000 |
| 9 | 500 | 500 | 500 | 500 | 500 | 1000 |
| 10 | 500 | 250 | 500 | 1000 | 500 | 1000 |
| 11 | 500 | 500 | 500 | 1000 | 500 | 1000 |
| 12 | 500 | 1000 | 1000 | 1000 | 500 | 500 |
| 13 | 250 | 500 | 500 | 500 | 1000 | 500 |
| 14 | 250 | 250 | 250 | 500 | 500 | 500 |
| Fluconazole | 1 | 2 | 1 | 2 | 2 | 2 |
| Compound | IC50 (µg/mL) | |
|---|---|---|
| MCF-7 Cell Line | NIH/3T3 Cell Line | |
| 1 | >500 | >500 |
| 2 | >500 | >500 |
| 3 | 500 | 250 |
| 4 | >500 | >500 |
| 5 | >500 | >500 |
| 6 | >500 | >500 |
| 7 | >500 | >500 |
| 8 | >500 | >500 |
| 9 | 102.58 | 86.33 |
| 10 | 121.79 | 250 |
| 11 | 125 | >500 |
| 12 | 500 | >500 |
| 13 | 500 | >500 |
| 14 | >500 | >500 |
| Cisplatin | 31.2 | 330.58 |
3. Experimental Section
3.1. General Information
3.2. Chemistry: General Procedures for the Synthesis of Compounds 1–14
3.2.1. Synthesis of 5-(4-Nitrophenyl)furfural thiosemicarbazone (A)/5-(4-Chloro-2-nitrophenyl)furfural thiosemicarbazone (B)
3.2.2. Synthesis of 2-[2-((5-(4-Nitrophenyl)furan-2-yl)methylene)hydrazinyl]-4-phenylthiazole/2-[2-((5-(4-chloro-2-nitrophenyl)furan-2-yl)methylene)hydrazinyl]-4-phenylthiazole Derivatives 1–14
3.3. Bioassays
3.3.1. Anticandidal Activity
3.3.2. Cytotoxicity
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silverman, R.B. The Organic Chemistry of Drug Design and Drug Action; Elsevier Academic Press: Burlington, MA, USA, 2004; pp. 298–302. [Google Scholar]
- Nussbaumer, S.; Bonnabry, P.; Veuthey, J.-L.; Fleury-Souverain, S. Analysis of anticancer drugs: A review. Talanta 2011, 85, 2265–2289. [Google Scholar] [CrossRef]
- Singh, S.; Saxena, A.K. 2D-QSAR of purine-scaffold novel class of Hsp90 binders that inhibit the proliferation of cancer cells. Med. Chem. Res. 2008, 17, 290–296. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef]
- Canuto, M.M.; Rodero, F.G. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2002, 2, 550–563. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive Candidiasis: A Persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Sheehan, D.J.; Hitchcock, C.A.; Ghannoum, M.A. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 2005, 18, 163–194. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar]
- Fluit, A.C.; Visser, M.R.; Schmitz, F.-J. Molecular detection of antimicrobial resistance. Clin. Microbiol. Rev. 2001, 14, 836–871. [Google Scholar] [CrossRef]
- Ahmad, I.; Beg, A.Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J. Ethnopharmacol. 2001, 74, 113–123. [Google Scholar] [CrossRef]
- Eicher, T.; Hauptmann, S. The Chemistry of Heterocycles, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003; pp. 149–154. [Google Scholar]
- Gupta, R.R.; Kumar, M.; Gupta, V. Heterocyclic Chemistry Volume II: Five-Membered Heterocycles; Springer: Berlin, Germany, 1999; pp. 416–417. [Google Scholar]
- Kashyap, S.J.; Garg, V.K.; Sharma, P.K.; Kumar, N.; Dudhe, R.; Gupta, J.K. Thiazoles: Having diverse biological activities. Med. Chem. Res. 2012, 21, 2123–2132. [Google Scholar] [CrossRef]
- Lu, Y.; Li, C.-M.; Wang, Z.; Ross, C.R.; Chen, J.; Dalton, J.T.; Li, W.; Miller, D.D. Discovery of 4-substituted methoxybenzoyl-aryl-thiazole as novel anticancer agents: Synthesis, biological evaluation, and structure-activity relationships. J. Med. Chem. 2009, 52, 1701–1711. [Google Scholar] [CrossRef]
- Lv, P.-C.; Li, D.-D.; Li, Q.-S.; Lu, X.; Xiao, Z.-P.; Zhu, H.-L. Synthesis, molecular docking and evaluation of thiazolyl-pyrazoline derivatives as EGFR TK inhibitors and potential anticancer agents. Bioorg. Med. Chem. Lett. 2011, 21, 5374–5377. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Qi, F.; Bensdorf, K.; Li, Z.; Zhang, H.; Qian, H.; Huang, W.; Cai, X.; Cao, P.; et al. Synthesis and biological activities of 2-amino-thiazole-5-carboxylic acid phenylamide derivatives. Arch. Pharm. Chem. Life Sci. 2011, 344, 451–458. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Brancale, A.; Ricci, A.; Hamel, E.; Bortolozzi, R.; Basso, G.; Viola, G. Convergent synthesis and biological evaluation of 2-amino-4-(3',4',5'-trimethoxyphenyl)-5-aryl thiazoles as microtubule targeting sgents. J. Med. Chem. 2011, 54, 5144–5153. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Z.; Zhuang, X.; Luo, J.; Cao, X.; Li, H.; Tu, Z.; Lu, X.; Ren, X.; Ding, K. New thiazole carboxamides as potent inhibitors of Akt kinases. Bioorg. Med. Chem. Lett. 2012, 22, 1208–1212. [Google Scholar] [CrossRef]
- Hassan, G.S.; El-Messery, S.M.; Al-Omary, F.A.M.; El-Subbagh, H.I. Substituted thiazoles VII. Synthesis and antitumor activity of certain 2-(substituted amino)-4-phenyl-1,3-thiazole analogs. Bioorg. Med. Chem. Lett. 2012, 22, 6318–6323. [Google Scholar]
- Popsavin, M.; Torović, L.; Svirčev, M.; Kojić, V.; Bogdanović, G.; Popsavin, V. Synthesis and antiproliferative activity of two new tiazofurin analogues with 2'-amido functionalities. Bioorg. Med. Chem. Lett. 2006, 16, 2773–2776. [Google Scholar] [CrossRef]
- Quada, J.C., Jr.; Boturyn, D.; Hecht, S.M. Photoactivated DNA cleavage by compounds structurally related to the bithiazole moiety of bleomycin. Bioorg. Med. Chem. 2001, 9, 2303–2314. [Google Scholar] [CrossRef]
- Easmon, J.; Heinisch, G.; Hofmann, J.; Langer, T.; Grunicke, H.H.; Fink, J.; Pürstinger, G. Thiazolyl and benzothiazolyl hydrazones derived from α-(N)-acetylpyridines and diazines: Synthesis, antiproliferative activity and CoMFA studies. Eur. J. Med. Chem. 1997, 32, 397–408. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef]
- Narang, R.; Narasimhan, B.; Sharma, S. A review on biological activities and chemical synthesis of hydrazide derivatives. Curr. Med. Chem. 2012, 19, 569–612. [Google Scholar] [CrossRef]
- Judge, V.; Narasimhan, B.; Ahuja, M. Isoniazid: The magic molecule. Med. Chem. Res. 2012, 21, 3940–3957. [Google Scholar] [CrossRef]
- Sah, P.P.T.; Peoples, S.A. Isonicotinyl hydrazones as antitubercular agents and derivatives for idendification of aldehydes and ketones. J. Am. Pharm. Assoc. 1954, 43, 513–524. [Google Scholar]
- Chakravarty, D.; Bose, A.; Bose, S. Synthesis and antitubercular activity of isonicotinoyl and cyanoacetyl hydrazones. J. Pharm. Sci. 1964, 53, 1036–1039. [Google Scholar] [CrossRef]
- Gürsoy, A.; Terzioglu, N.; Ötük, G. Synthesis of some new hydrazide-hydrazones, thiosemicarbazides and thiazolidinones as possible antimicrobials. Eur. J. Med. Chem. 1997, 32, 753–757. [Google Scholar]
- Vicini, P.; Zani, F.; Cozzini, P.; Doytchinova, I. Hydrazones of 1,2-benzisothiazole hydrazides: Synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2002, 37, 553–564. [Google Scholar] [CrossRef]
- Kumar, P.; Narasimhan, B.; Sharma, D.; Judge, V.; Narang, R. Hansch analysis of substituted benzoic acid benzylidene/furan-2-yl-methylene hydrazides as antimicrobial agents. Eur. J. Med. Chem. 2009, 44, 1853–1863. [Google Scholar] [CrossRef]
- Terzioğlu, N.; Gürsoy, A. Synthesis and anticancer evaluation of some new hydrazone derivatives of 2,6-dimethylimidazo[2,1-b][1,3,4]thiadiazole-5-carbohydrazide. Eur. J. Med. Chem. 2003, 38, 781–786. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Y.; Zhai, X.; Wang, L.; Yang, J.; Tan, Z.; Gong, P. Design, Synthesis and anticancer activities of diaryl urea derivatives bearing N-acylhydrazone moiety. Chem. Pharm. Bull. 2012, 60, 1046–1054. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, C.-D.; Zhao, B.-X.; Zhao, J.; Shin, D.-S.; Miao, J.-Y. Synthesis and structure–activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide hydrazone derivatives as potential agents against A549 lung cancer cells. Eur. J. Med. Chem. 2008, 43, 2347–2353. [Google Scholar] [CrossRef]
- Guay, D.R. An update on the role of nitrofurans in the management of urinary tract infections. Drugs 2001, 61, 353–364. [Google Scholar] [CrossRef]
- Holla, B.S.; Malini, K.V.; Rao, B.S.; Sarojini, B.K.; Kumari, N.S. Synthesis of some new 2,4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agents. Eur. J. Med. Chem. 2003, 38, 313–318. [Google Scholar] [CrossRef]
- Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard, M27-A2; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2002.
- Özdemir, A.; Turan-Zitouni, G.; Kaplancıklı, Z.A.; İşcan, G.; Khan, S.; Demirci, F. Synthesis and the selective antifungal activity of 5,6,7,8-tetrahydroimidazo[1,2-a]pyridine derivatives. Eur. J. Med. Chem. 2010, 45, 2080–2084. [Google Scholar]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar]
- Sample Availability: Samples of the compounds 1–14 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Altıntop, M.D.; Özdemir, A.; Turan-Zitouni, G.; Ilgın, S.; Atlı, Ö.; Demirci, F.; Kaplancıklı, Z.A. Synthesis and in Vitro Evaluation of New Nitro-Substituted Thiazolyl Hydrazone Derivatives as Anticandidal and Anticancer Agents. Molecules 2014, 19, 14809-14820. https://doi.org/10.3390/molecules190914809
Altıntop MD, Özdemir A, Turan-Zitouni G, Ilgın S, Atlı Ö, Demirci F, Kaplancıklı ZA. Synthesis and in Vitro Evaluation of New Nitro-Substituted Thiazolyl Hydrazone Derivatives as Anticandidal and Anticancer Agents. Molecules. 2014; 19(9):14809-14820. https://doi.org/10.3390/molecules190914809
Chicago/Turabian StyleAltıntop, Mehlika Dilek, Ahmet Özdemir, Gülhan Turan-Zitouni, Sinem Ilgın, Özlem Atlı, Fatih Demirci, and Zafer Asım Kaplancıklı. 2014. "Synthesis and in Vitro Evaluation of New Nitro-Substituted Thiazolyl Hydrazone Derivatives as Anticandidal and Anticancer Agents" Molecules 19, no. 9: 14809-14820. https://doi.org/10.3390/molecules190914809
APA StyleAltıntop, M. D., Özdemir, A., Turan-Zitouni, G., Ilgın, S., Atlı, Ö., Demirci, F., & Kaplancıklı, Z. A. (2014). Synthesis and in Vitro Evaluation of New Nitro-Substituted Thiazolyl Hydrazone Derivatives as Anticandidal and Anticancer Agents. Molecules, 19(9), 14809-14820. https://doi.org/10.3390/molecules190914809