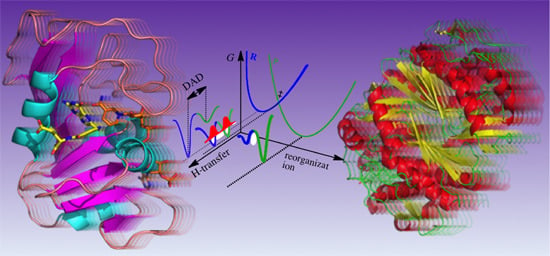
Linking Protein Motion to Enzyme Catalysis
Abstract
Share and Cite
Singh, P.; Abeysinghe, T.; Kohen, A. Linking Protein Motion to Enzyme Catalysis. Molecules 2015, 20, 1192-1209. https://doi.org/10.3390/molecules20011192
Singh P, Abeysinghe T, Kohen A. Linking Protein Motion to Enzyme Catalysis. Molecules. 2015; 20(1):1192-1209. https://doi.org/10.3390/molecules20011192
Chicago/Turabian StyleSingh, Priyanka, Thelma Abeysinghe, and Amnon Kohen. 2015. "Linking Protein Motion to Enzyme Catalysis" Molecules 20, no. 1: 1192-1209. https://doi.org/10.3390/molecules20011192
APA StyleSingh, P., Abeysinghe, T., & Kohen, A. (2015). Linking Protein Motion to Enzyme Catalysis. Molecules, 20(1), 1192-1209. https://doi.org/10.3390/molecules20011192