A Synergistic, Balanced Antioxidant Cocktail, Protects Aging Rats from Insulin Resistance and Absence of Meal-Induced Insulin Sensitization (AMIS) Syndrome
Abstract
:1. Introduction
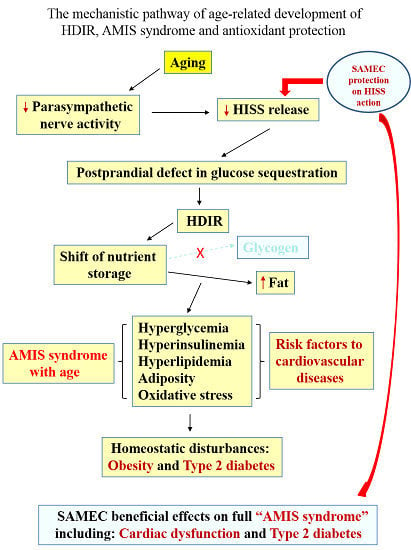
Aging and Age-Related Metabolic Dysfunctions: The Role of HISS and AMIS
2. Summary of the HISS Story
HISS-Dependent Insulin Resistance (HDIR)
3. The AMIS Syndrome
4. Aging, HISS and HDIR
4.1. Aging and HISS Release
4.2. The Aging Model of HDIR

4.3. Cardiac Dysfunction
5. Antioxidant Protection of Aging Animals
5.1. Development of a Synergistic Balanced Antioxidant Cocktail SAMEC (S-adenosylmethionine, Vitamins E and C)
5.2. SAMEC Protection from Acute Liver Injury
5.3. SAMEC Protection in a Low-Dose Sucrose Supplemented Model of Accelerated Development of AMIS
5.4. Cardiac Protection Conferred by SAMEC
5.5. Physical Exercise and SAMEC Effects on AMIS
6. Conclusions

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Drucker, R.P. The Code of Life; Health Empowerment Publications LLC: Wilmington, DE, USA, 2012; pp. 67–68. [Google Scholar]
- De la Torre-Ruiz, M.A.; Pujol, N.; Sundaram, V. Coping with Oxidative Stress. The Yeast Model. Curr. Drug Targets 2014, 15. [Google Scholar] [CrossRef]
- De Meersman, R.E.; Stein, P.K. Vagal modulation and aging. Biol. Psychol. 2007, 74, 165–173. [Google Scholar]
- Bertolotti, M.; Lonardo, A.; Mussi, C.; Baldelli, E.; Pellegrini, E.; Ballestri, S.; Romagnoli, D.; Loria, P. Nonalcoholic fatty liver disease and aging: Epidemiology to management. World J. Gastroenterol. 2014, 20, 14185–14204. [Google Scholar]
- Saeidnia, S.; Abdollahi, M. Antioxidants: friends or foe in prevention or treatment of cancer: The debate of the century. Toxicol. Appl. Pharmacol. 2013, 271, 49–63. [Google Scholar]
- Godic, A.; Poljsak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell. Longev. 2014. [Google Scholar] [CrossRef]
- Ming, Z.; Legare, D.J.; Lautt, W.W. Obesity, syndrome X, and diabetes: The role of HISS-dependent insulin resistance altered by sucrose, an antioxidant cocktail, and age. Can. J. Physiol. Pharmacol. 2009, 87, 873–882. [Google Scholar]
- Ming, Z.; Legare, D.J.; Lautt, W.W. Absence of meal-induced insulin sensitization (AMIS) in aging rats is associated with cardiac dysfunction that is protected by antioxidants. J. Appl. Physiol. 2011, 111, 704–714. [Google Scholar]
- Lautt, W.W.; Ming, Z. Use of an antioxidant cocktail for insulin resistance associated with age and a high sugar diet: A hepatic mechanism. In Nutrition, Diet Therapy, and the Liver; Preedy, V.R., Srirajaskanthan, R., Lakshman, R., Watson, R.R., Eds.; Taylor and Francis, CRC Press: London, UK, 2010; pp. 319–333. [Google Scholar]
- Chowdhury, K.K.; Legare, D.J.; Lautt, W.W. Exercise enhancement of hepatic insulin-sensitising substance-mediated glucose uptake in diet-induced prediabetic rats. Br. J. Nutr. 2013, 109, 844–852. [Google Scholar]
- Chowdhury, K.K.; Legare, D.J.; Lautt, W.W. Insulin sensitization by voluntary exercise in aging rats is mediated through hepatic insulin sensitizing substance (HISS). Exp. Gerontol. 2011, 46, 73–80. [Google Scholar]
- Chowdhury, K.K.; Legare, D.J.; Lautt, W.W. Lifestyle impact on meal-induced insulin sensitization in health and prediabetes: A focus on diet, antioxidants, and exercise interventions. Can. J. Physiol. Pharmacol. 2013, 91, 91–100. [Google Scholar]
- Ming, Z.; Fan, Y.J.; Yang, X.; Lautt, W.W. Synergistic protection by S-adenosylmethionine with vitamins C and E on liver injury induced by thioacetamide in rats. Free Radic. Biol. Med. 2006, 40, 617–624. [Google Scholar]
- Lautt, W.W. State of the Art 1997: Hepatic parasympathetic nerves and glucose metabolism. In Liver and Nervous System; Häussinger, D., Jungermann, K., Eds.; Kluwer Academic Publishers: London, UK, 1998; pp. 1–14. [Google Scholar]
- Latour, M.G.; Lautt, W.W. Insulin sensitivity regulated by feeding in the conscious unrestrained rat. Can. J. Physiol. Pharmacol. 2002, 80, 8–12. [Google Scholar]
- Patarrao, R.S.; Lautt, W.W.; Guarino, M.P.; Afonso, R.A.; Ribeiro, R.T.; Fernandes, A.B.; Boavida, J.M.; Macedo, M.P. A new technique to assess insulin sensitivity in humans: The rapid insulin sensitivity test (RIST). Proc. West. Pharmacol. Soc. 2007, 50, 105–109. [Google Scholar]
- Lautt, W.W.; Wang, H.H. Obesity as an Early Symptom of the AMIS Syndrome. J. Clin. Med. 2014, 3, 1178–1198. [Google Scholar]
- Lautt, W.W. The HISS story overview: A novel hepatic neurohumoral regulation of peripheral insulin sensitivity in health and diabetes. Can. J. Physiol. Pharmacol. 1999, 77, 553–562. [Google Scholar]
- Fernandes, A.B.; Patarrao, R.S.; Videira, P.A.; Macedo, M.P. Understanding postprandial glucose clearance by peripheral organs: The role of the hepatic parasympathetic system. J. Neuroendocrinol. 2011, 23, 1288–1295. [Google Scholar]
- Sadri, P.; Reid, M.A.; Afonso, R.A.; Schafer, J.; Legare, D.J.; Macedo, P.M.; Lautt, W.W. Meal-induced insulin sensitization in conscious and anaesthetized rat models comparing liquid mixed meal with glucose and sucrose. Br. J. Nutr. 2006, 95, 288–295. [Google Scholar]
- Patarrao, R.S.; Lautt, W.W.; Afonso, R.A.; Ribeiro, R.T.; Guarino, M.P.; Fernandes, A.B.; Boavida, J.M.; Macedo, M.P. Meal-induced insulin sensitization and its parasympathetic regulation in humans. Can. J. Physiol. Pharmacol. 2008, 86, 880–888. [Google Scholar]
- Sadri, P.; Lautt, W.W. Blockade of hepatic nitric oxide synthase causes insulin resistance. Am. J. Physiol. 1999, 277, G101–G108. [Google Scholar]
- Guarino, M.P.; Correia, N.C.; Lautt, W.W.; Macedo, M.P. Insulin sensitivity is mediated by the activation of the ACh/NO/cGMP pathway in rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G527–G532. [Google Scholar]
- Tateishi, N.; Higashi, T.; Naruse, A.; Nakashima, K.; Shiozaki, H. Rat liver glutathione: Possible role as a reservoir of cysteine. J. Nutr. 1977, 107, 51–60. [Google Scholar]
- Guarino, M.P.; Afonso, R.A.; Raimundo, N.; Raposo, J.F.; Macedo, M.P. Hepatic glutathione and nitric oxide are critical for hepatic insulin-sensitizing substance action. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G588–G594. [Google Scholar]
- Lautt, W.W.; Schafer, J.; Macedo, M.P.; Legare, D.J. Bethanechol and N-acetylcysteine mimic feeding signals and reverse insulin resistance in fasted and sucrose-induced diabetic rats. Can. J. Physiol. Pharmacol. 2011, 89, 135–142. [Google Scholar]
- Guarino, M.P.; Macedo, M.P. Co-administration of glutathione and nitric oxide enhances insulin sensitivity in Wistar rats. Br. J. Pharmacol. 2006, 147, 959–965. [Google Scholar]
- Lautt, W.W.; Macedo, M.P.; Legare, D.J.; Reid, M.A.G.; Guarino, M. Pharmaceutical reversal of meal-induced insulin resistance. Proc. West. Pharmacol. Soc. 2004, 47, 30–32. [Google Scholar]
- Lautt, W.W. New paradigm for the mechanism and treatment of insulin resistance. Proc. West. Pharmacol. Soc. 2002, 45, 223–224. [Google Scholar]
- Xie, H.; Lautt, W.W. Insulin resistance caused by hepatic cholinergic interruption and reversed by acetylcholine administration. Am. J. Physiol. 1996, 271, E587–E592. [Google Scholar]
- Xie, H.; Lautt, W.W. Insulin resistance of skeletal muscle produced by hepatic parasympathetic interruption. Am. J. Physiol. 1996, 270, E858–E863. [Google Scholar]
- Xie, H.; Lautt, W.W. Insulin resistance produced by hepatic denervation or muscarinic cholinergic blockade. Proc. West. Pharmacol. Soc. 1994, 37, 39–40. [Google Scholar]
- Xie, H.; Lautt, W.W. M1 muscarinic receptor blockade causes insulin resistance in the cat. Proc. West. Pharmacol. Soc. 1995, 38, 83–84. [Google Scholar]
- Sadri, P.; Legare, D.J.; Lautt, W.W. Insulin resistance caused by nitric oxide synthase inhibition. Proc. West. Pharmacol. Soc. 1997, 40, 19–20. [Google Scholar]
- Sadri, P.; Lautt, W.W. Blockade of nitric oxide production in the liver causes insulin resistance. Proc. West. Pharmacol. Soc. 1998, 41, 37–38. [Google Scholar]
- Lautt, W.W. A proposed new paradigm for insulin resistance. Metab. Syndr. Relat. Disord. 2003, 1, 261–270. [Google Scholar]
- Lautt, W.W.; Legare, D.J.; Reid, M.A.G.; Sadri, P.; Ting, J.W.; Prieditis, H. Alcohol suppresses meal-induced insulin sensitization (MIS). Metab. Syndr. Relat. Disord. 2005, 3, 51–59. [Google Scholar]
- Afonso, R.A.; Lautt, W.W.; Ribeiro, R.T.; Legare, D.J.; Macedo, M.P. Insulin resistance in two animal models of obesity: A comparison of HISS-dependent and HISS-independent insulin action in high-fat diet-fed and Zucker rats. Proc. West. Pharmacol. Soc. 2007, 50, 110–114. [Google Scholar]
- Afonso, R.A.; Ribeiro, R.T.; Macedo, M.P. Defective hepatic nitric oxide action results in HISS-dependent insulin resistance in spontaneously hypertensive rats. Proc. West. Pharmacol. Soc. 2004, 47, 103–104. [Google Scholar]
- Lautt, W.W.; Ming, Z.; Legare, D.J. Attenuation of age- and sucrose-induced insulin resistance and syndrome X by a synergistic antioxidant cocktail: The AMIS syndrome and HISS hypothesis. Can. J. Physiol. Pharmacol. 2010, 88, 313–323. [Google Scholar]
- Lautt, W.W.; Ming, Z.; Macedo, M.P.; Legare, D.J. HISS-dependent insulin resistance (HDIR) in aged rats is associated with adiposity, progresses to syndrome X, and is attenuated by a unique antioxidant cocktail. Exp. Gerontol. 2008, 43, 790–800. [Google Scholar]
- Ribeiro, R.T.; Afonso, R.A.; Guarino, M.P.; Macedo, M.P. Loss of postprandial insulin sensitization during aging. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 560–565. [Google Scholar]
- Ribeiro, R.T.; Lautt, W.W.; Legare, D.J.; Macedo, M.P. Insulin resistance induced by sucrose feeding in rats is due to an impairment of the hepatic parasympathetic nerves. Diabetologia 2005, 48, 976–983. [Google Scholar]
- Seredycz, L.I.; Ming, Z.; Lautt, W.W. Acute hemorrhage causes hepatic insulin sensitizing substance (HISS)-dependent insulin resistance. Can. J. Physiol. Pharmacol. 2006, 84, 1145–1151. [Google Scholar]
- Lautt, W.W. Practice and principles of pharmacodynamic determination of HISS-dependent and HISS-independent insulin action: Methods to quantitate mechanisms of insulin resistance. Med. Res. Rev. 2003, 23, 1–14. [Google Scholar]
- Sadri, P.; Legare, D.J.; Takayama, S.; Lautt, W.W. Increased incidence of HISS-dependent insulin resistance in female rats prenatally exposed to ethanol. Can. J. Physiol. Pharmacol. 2005, 83, 383–387. [Google Scholar]
- Ming, Z.; Lautt, W.W. HISS, not insulin, causes vasodilation in response to administered insulin. J. Appl. Physiol. 2011, 110, 60–68. [Google Scholar]
- Facchini, F.S.; Hua, N.; Abbasi, F.; Reaven, G.M. Insulin resistance as a predictor of age-related diseases. J. Clin. Endocrinol. Metab. 2001, 86, 3574–3578. [Google Scholar]
- Honek, J.; Seki, T.; Iwamoto, H.; Fischer, C.; Li, J.; Lim, S.; Samani, N.J.; Zang, J.; Cao, Y. Modulation of age-related insulin sensitivity by VEGF-dependent vascular plasticity in adipose tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 14906–14911. [Google Scholar]
- Moller, D.E.; Kaufman, K.D. Metabolic syndrome: A clinical and molecular perspective. Annu. Rev. Med. 2005, 56, 45–62. [Google Scholar]
- Johnson, A.M.; Olefsky, J.M. The origins and drivers of insulin resistance. Cell 2013, 152, 673–684. [Google Scholar]
- Park, M.H.; Kim, D.H.; Lee, E.K.; Kim, N.D.; Im, D.S.; Lee, J.; Yu, B.P.; Chung, H.Y. Age-related inflammation and insulin resistance: A review of their intricate interdependency. Arch. Pharm. Res. 2014, 37, 1507–1514. [Google Scholar]
- De Tata, V. Age-related impairment of pancreatic Beta-cell function: Pathophysiological and cellular mechanisms. Front. Endocrinol. (Lausanne) 2014, 5, 138. [Google Scholar]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar]
- Sepe, A.; Tchkonia, T.; Thomou, T.; Zamboni, M.; Kirkland, J.L. Aging and regional differences in fat cell progenitors—A mini-review. Gerontology 2011, 57, 66–75. [Google Scholar]
- Lautt, W.W.; Wang, X.; Sadri, P.; Legare, D.J.; Macedo, M.P. Rapid insulin sensitivity test (RIST). Can. J. Physiol. Pharmacol. 1998, 76, 1080–1086. [Google Scholar]
- Davidson, M.B. The effect of aging on carbohydrate metabolism: A review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism 1979, 28, 688–705. [Google Scholar]
- Buwalda, B.; Strubbe, J.H.; Hoes, M.W.; Bohus, B. Reduced preabsorptive insulin response in aged rats: Differential effects of amphetamine and arginine-vasopressin. J. Auton. Nerv. Syst. 1991, 36, 123–128. [Google Scholar]
- Espinola, E.B.; Oliveira, M.G.; Carlini, E.A. Differences in central and peripheral responses to oxotremorine in young and aged rats. Pharmacol. Biochem. Behav. 1999, 62, 419–423. [Google Scholar]
- Rowe, J.W.; Minaker, K.L.; Pallotta, J.A.; Flier, J.S. Characterization of the insulin resistance of aging. J. Clin. Investig. 1983, 71, 1581–1587. [Google Scholar]
- Lautt, W.W. Postprandial insulin resistance as an early predictor of cardiovascular risk. Ther. Clin. Risk Manag. 2007, 3, 761–770. [Google Scholar]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar]
- Sastre, J.; Pallardo, F.V.; Vina, J. The role of mitochondrial oxidative stress in aging. Free Radic. Biol. Med. 2003, 35, 1–8. [Google Scholar]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar]
- Esteve, J.M.; Mompo, J.; Garcia de la Asuncion, J.; Sastre, J.; Asensi, M.; Boix, J.; Vina, J.R.; Vina, J.; Pallardo, F.V. Oxidative damage to mitochondrial DNA and glutathione oxidation in apoptosis: Studies in vivo and in vitro. FASEB J. 1999, 13, 1055–1064. [Google Scholar]
- Rebrin, I.; Bayne, A.C.; Mockett, R.J.; Orr, W.C.; Sohal, R.S. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem. J. 2004, 382, 131–136. [Google Scholar]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar]
- Hunter, A.L.; Holscher, M.A.; Neal, R.A. Thioacetamide-induced hepatic necrosis. I. Involvement of the mixed-function oxidase enzyme system. J. Pharmacol. Exp. Ther. 1977, 200, 439–448. [Google Scholar]
- Porter, W.R.; Neal, R.A. Metabolism of thioacetamide and thioacetamide S-oxide by rat liver microsomes. Drug Metab. Dispos. 1978, 6, 379–388. [Google Scholar]
- Chowdhury, K.K.; Legare, D.J.; Lautt, W.W. Interaction of antioxidants and exercise on insulin sensitivity in healthy and prediabetic rats. Can. J. Physiol. Pharmacol. 2013, 91, 570–577. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.H.; Chowdhury, K.K.; Lautt, W.W. A Synergistic, Balanced Antioxidant Cocktail, Protects Aging Rats from Insulin Resistance and Absence of Meal-Induced Insulin Sensitization (AMIS) Syndrome. Molecules 2015, 20, 669-682. https://doi.org/10.3390/molecules20010669
Wang HH, Chowdhury KK, Lautt WW. A Synergistic, Balanced Antioxidant Cocktail, Protects Aging Rats from Insulin Resistance and Absence of Meal-Induced Insulin Sensitization (AMIS) Syndrome. Molecules. 2015; 20(1):669-682. https://doi.org/10.3390/molecules20010669
Chicago/Turabian StyleWang, Hui Helen, Kawshik K. Chowdhury, and W. Wayne Lautt. 2015. "A Synergistic, Balanced Antioxidant Cocktail, Protects Aging Rats from Insulin Resistance and Absence of Meal-Induced Insulin Sensitization (AMIS) Syndrome" Molecules 20, no. 1: 669-682. https://doi.org/10.3390/molecules20010669
APA StyleWang, H. H., Chowdhury, K. K., & Lautt, W. W. (2015). A Synergistic, Balanced Antioxidant Cocktail, Protects Aging Rats from Insulin Resistance and Absence of Meal-Induced Insulin Sensitization (AMIS) Syndrome. Molecules, 20(1), 669-682. https://doi.org/10.3390/molecules20010669