Phytochemical and Biological Investigation of Two Diplotaxis Species Growing in Tunisia: D. virgata & D. erucoides
Abstract
:1. Introduction
2. Results and Discussion
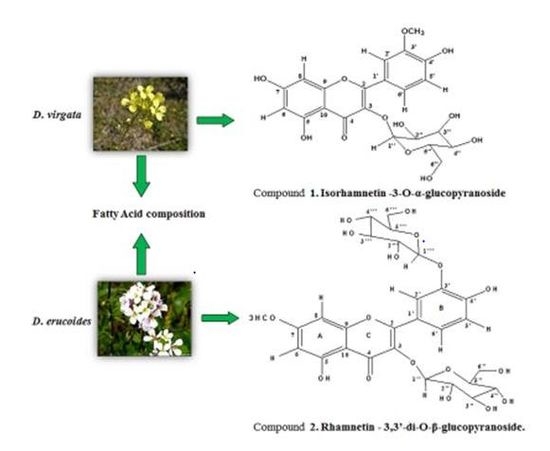
2.1. Structure Determination

| Position | δ (ppm) | COSY | HMBC | NOESY | |
|---|---|---|---|---|---|
| 1H, mult. (J in Hz) | 13C | ||||
| Aglycon | |||||
| 2 | 159.5 | ||||
| 3 | 136 | ||||
| 4 | 179.9 | ||||
| 5 | 162.8 | ||||
| 6 | 6.14 s | 100.9 | 8 | 10, 8, 7, 5 | |
| 7 | 165.9 | ||||
| 8 | 6.28 s | 96.1 | 6 | 10, 9, 7, 6 | |
| 9 | 158.7 | ||||
| 10 | 106.5 | ||||
| 1ʹ | 123.7 | ||||
| 2ʹ | 7.73 s | 115 | 6ʹ | 1ʹ, 3ʹ, 4ʹ, 2, 6ʹ | 3ʹ-OMe |
| 3ʹ | 149 | ||||
| 4ʹ | 151 | ||||
| 5ʹ | 6.88 d (J = 8.1) | 116.9 | 1ʹ, 3ʹ, 4ʹ | 6ʹ | |
| 6ʹ | 7.48 d (J = 7.8) | 124.8 | 6ʹ | 4ʹ, 2ʹ, 2 | 5ʹ |
| 3ʹ-OMe | 3.87 s | 57.8 | 5ʹ, 2ʹ | 3ʹ | 2ʹ |
| Glc | |||||
| 1ʹʹ | 5.17 d (J = 4.5) | 104.4 | 2ʹʹ | 3 | 3, 2ʹʹ |
| 2ʹʹ | 3.48 m | 76.4 | 1ʹʹ | 3ʹʹ | |
| 3ʹʹ | 3.48 m | 78.2 | 4ʹʹ | 4ʹʹ, 2ʹʹ | |
| 4ʹʹ | 3.34 m | 71.8 | 5ʹʹ | 3ʹʹ | |
| 5ʹʹ | 3.25 m | 78.7 | 6aʹʹ | 4ʹʹ | |
| 6aʹʹ | 3.54 m | 63 | 5ʹʹ, 6bʹʹ | ||
| 6bʹʹ | 3.66 m | 63 | 6aʹʹ | ||

| Position | δ (ppm) | COSY | HMBC | NOESY | |
|---|---|---|---|---|---|
| 1H, mult. (J in Hz) | 13C | ||||
| Aglycon | |||||
| 2 | 160 | ||||
| 3 | 136.7 | ||||
| 4 | 180.6 | ||||
| 5 | 163 | ||||
| 6 | 6.34 s | 100.3 | 8 | 10, 8, 7, 5 | 7-OMe |
| 7 | 168.2 | ||||
| 8 | 6.64 s | 94.5 | 6 | 10, 9, 7, 6 | 7-OMe |
| 9 | 159.2 | ||||
| 10 | 107.5 | ||||
| 1ʹ | 124.4 | ||||
| 2ʹ | 7.99 d (J = 2.1) | 121 | 6ʹ | 6ʹ, 3ʹ, 4ʹ, 2 | |
| 3ʹ | 147.1 | ||||
| 4ʹ | 152.3 | ||||
| 5ʹ | 7.01 d (J = 8.6) | 118.2 | 6ʹ | 1ʹ, 4ʹ, 3ʹ | 6ʹ |
| 6ʹ | 7.81 dd (J = 8.6, 2.1) | 128 | 5ʹ, 2ʹ | 4ʹ | 5ʹ |
| 7-OCH3 | 3.87 s | 58.7 | 7 | 6, 8 | |
| (Glc I) | |||||
| 1ʹʹ | 5.11 d (J = 7.6) | 104.8 | 2ʹʹ | 3 | |
| 2ʹʹ | 3.48 m | 76.4 | 1ʹʹ | ||
| 3ʹʹ | 3.43 m | 78.5 | |||
| 4ʹʹ | 3.34 m | 71.9 | |||
| 5ʹʹ | 3.19 m | 79.1 | 6aʹʹ, 6bʹʹ | ||
| 6aʹʹ | 3.52 m | 63.2 | 5ʹʹ | ||
| 6bʹʹ | 3.64 m | 63.2 | 5ʹʹ | ||
| (Glc II) | |||||
| 1ʹʹʹ | 4.95 d (J = 7.4) | 105.2 | 2ʹʹʹ | 3ʹ | |
| 2ʹʹʹ | 3.58 m | 78.1 | 1ʹʹʹ | ||
| 3ʹʹʹ | 3.47 m | 71.9 | 4ʹʹʹ | ||
| 4ʹʹʹ | 3.58 m | 75.6 | 5ʹʹʹ | ||
| 5ʹʹʹ | 3.47 m | 79 | 6aʹʹʹ,6bʹʹʹ | ||
| 6aʹʹʹ | 3.77 m | 63.2 | 5ʹʹʹ, 6bʹʹʹ | ||
| 6bʹʹʹ | 3.91 m | 63.2 | 5ʹʹʹ, 6aʹʹʹ | ||
2.2. Fatty Acid Composition (GC-MS)
| Fatty Acid | HFF-DV (%) | HFPA-DE (%) |
|---|---|---|
| Palmitic acid C16:0 | 18.23 | 14.35 |
| Stearic acid C18:0 | 3.08 | - |
| Oleic acid C18:1 | 3.42 | - |
| Linoleic acid C18: 2 | 23.65 | 29.1 |
| Linolelaidic acid C18: 2 | 15.66 | 6.91 |
2.3. Antioxidant Activity
2.3.1. DPPH Radical Scavenging Activity
2.3.2. ABTS Radical Scavenging Activity
| Samples | DPPH Radical a | ABTS+ Radical a |
|---|---|---|
| IC50 b | IC50 | |
| Flowers(D. virgata) | ||
| EtOH extract | 26.03 ± 1.42 | 31.80 ± 1.12 |
| EtOAc fraction | 40.05 ± 1.78 | 44.00 ± 1.72 |
| n-BuOH fraction | 20.01 ± 0.32 | 25.54 ± 1.32 |
| Compound 1 | 16.01 ± 0.13 | 17.03 ± 0.02 |
| Aerial parts(D. erucoides) | ||
| EtOH extract | 27.02 ± 1.53 | 27.04 ± 1.53 |
| EtOAc fraction | 26.03 ± 1.61 | 32.06 ± 1.63 |
| n-BuOH fraction | 24.01 ± 1.02 | 28.05 ± 1.43 |
| Compound 2 | 18.00 ± 0.01 | 19.04 ± 0.02 |
| BHT c | 18.00 ± 0.23 | 50.00 ± 0.20 |
| Quercetin d | 5.00 ± 0.12 | 6.91 ± 0.42 |
2.4. Antibacterial Activity
| Bacterial Strains | Inhibition Diameter a (IZ in mm), Concentration = 250 µg/mL | ||||
|---|---|---|---|---|---|
| n-BuOH (D. virgata) | Compound 1 | n-BuOH (D. erucoides) | Compound 2 | Ampicillin b | |
| Gram + Bacteria | |||||
| Listeria monocytogenes ATCC 11120 | 20.00 ± 0.01 | 13.60 ± 0.04 | 16.02 ± 0.03 | 14.50 ± 0.01 | 16.00 ± 0.01 |
| Staphylococcus aureus ATCC 25923 | 19.04 ± 0.05 | 12.04 ± 0.05 | 20.04 ± 0.01 | 13.00 ± 0.02 | 18.00 ± 0.03 |
| Gram − Bacteria | |||||
| Aeromona hydrophila ATCC 1943 | 21.00 ± 0.05 | 16.00 ± 0.01 | 21.07 ± 0.05 | 17.50 ± 0.05 | 17.00 ± 0.02 |
| Pseudomonas aeruginosa ATCC 9027 | 15.00 ± 0.02 | 13.00 ± 0.03 | 20.00 ± 0.02 | 17.00 ± 0.05 | 19.00 ± 0.01 |
| Salmonella enteritidis ATCC 14028 | 15.03 ± 0.01 | 12.50 ± 0.05 | 18.00 ± 0.02 | 14.00 ± 0.03 | 19.00 ± 0.04 |
| Escherichia coli ATCC 25922 | 22.03 ± 0.02 | 16.55 ± 0.05 | 16.00 ± 0.01 | 17.60 ± 0.04 | 20.00 ± 0.03 |
| Klebsiella pneumonia ATCC 13833 | 16.00 ± 0.03 | 14.00 ± 0.01 | 17.06 ± 0.02 | 15.00 ± 0.02 | 21.00 ± 0.02 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Fatty Acid Determination (GC-MS)
3.5. Antioxidant Activity
3.5.1. DPPH Assay
3.5.2. ABTS Test
3.6. Antimicrobial Activity by Wells Method
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- D’Antuono, L.F.; Elementi, S.; Neri, R. Glucosinolates in Diplotaxis and Eruca leaves: Diversity, taxonomic relations and applied aspects. Phytochemistry 2008, 69, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Pottier-Alapetite, G. Flore de la Tunisie: Angiospermes-Dicotyledones, Apétales-Dialypétales; (In French). Official printing: Tunis, Tunisia, 1979; pp. 195–198. [Google Scholar]
- Tlig, T.; Gorai, M.; Neffati, M. Germination responses of Diplotaxis harra to temperature and salinity. Flora. 2008, 203, 421–428. [Google Scholar] [CrossRef]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef]
- Pieroni, A.; Quave, C.L.; Santoro, R.F. Folk pharmaceutical knowledge in the territory of the Dolomiti Lucane, inland southern Italy. J. Ethnopharmacol. 2004, 95, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, M.L.; Corradi, L. Ethnopharmacobotanical remarks on the Province of Chieti town (Abruzzo, Central Italy). J. Ethnopharmacol. 2001, 74, 17–40. [Google Scholar] [CrossRef]
- Serdal Sakcali, M.; Serin, M. Seed germination behaviour of Diplotaxis tenuifolia. Eur. Asia J. Bio. Sci. 2009, 3, 107–112. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Crozier, A. Dietary Flavonoids and Phenolic Compounds; Fraga, C.G., Ed.; John Wiley & Sons: New Jersey, NJ, USA; pp. 5–18.
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutrition Res. 2004, 24, 851–874. [Google Scholar]
- Fernández-López, J.; Zhi, N.; Aleson-Carbonell, L.; Pérez-Alvarez, J.A.; Kuri, V. Antioxidant and antibacterial activities of natural extracts application in beefmeatballs. Meat Sci. 2005, 69, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Botterweck, A.A.; Verhagen, H.; Goldbohm, R.A.; Kleinjans, J.; Brandt, P.A. Intake of butylated hydroxyanisole and butylated hydroxytoluene andstomach cancer risk: Results from analyses in the Netherlands Cohort study. Food Chem. Toxicol. 2000, 387, 599–605. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005, 26, 343–356. [Google Scholar]
- Goda, Y.; Hoshin, K.; Akilyama, H.; Ishikawa, T.; Abe, Y.; Nakamura, T.; Otsuka, H.; Takeda, Y.; Tanimura, A.; Toyoda, M. Constituents in Watercress: Inhibitions of Histamine Release from RBL-2H3 Cells Induced by Antigen Stimulation. Biol. Pharm. Bull. 1999, 22, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Nørbæk, R.; Nielsen, J.K.; Kondo, T. Flavonoids from owers of two Crocus chrysanthus-biflorus cultivars: Eye-catcher and Spring Pearl (Iridaceae). Phytochemistry 1999, 51, 1139–1146. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, X.-F.; Yan, B.Z.; Shi, H.-M.; Du, L.-B.; Zhang, Y.-Z.; Wang, L.-F.; Tang, Y.-L.; Liu, Y. A novel flavonoid from Lespedeza virgata (Thunb.) DC.: Structural elucidation and antioxidative activity. Bioorg. Med. Chem. Letters. 2007, 17, 6311–6315. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Liu, J.; He, D.-H.; Xu, H.-X.; Ding, L.-S.; Bao, W.-K.; Zhou, Z.-Q.; Zhou, Y. Three new phenolic compounds from the leaves of Rosa sericea. Fitoterapia 2013, 84, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.H.; Priyadarsini, K.I.; Satav, J.G.; Banavalikar, M.M.; Sohoni, P.P.; Biyani, M.K. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry 2003, 63, 97–104. [Google Scholar] [CrossRef]
- Falleh, H.; Msilini, N.; Oueslati, S.; Ksouri, R.; Magne, C.; Lachaal, M.; Karray Bouraoui, N. Diplotaxis harra and Diplotaxis simplex organs: Assessment of phenolics and biological activities before and after fractionation. Ind. Crop. Prod. 2013, 45, 141–147. [Google Scholar] [CrossRef]
- Lee, J.; Renita, M.; Fioritto, R.J.; St Martin, S.K.; Schwartz, S.J.; Vodovotz, Y. Isoflavone characterization and antioxidant activity of ohio soybeans. J. Agric. Food Chem. 2004, 52, 2647–2651. [Google Scholar] [CrossRef] [PubMed]
- Pengfei, L.; Tiansheng, D.; Xianglin, H.; Jianguo, W. Antioxidant properties of isolated isorhamnetin from the sea buckthorn marc. Plant Foods Hum. Nutr. 2009, 64, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhan, W.Q.; Liu, X.; Jiang, S.X. Antioxidant activities of extracts and flavonoid compounds from Oxytropis falcate Bunge. Nat. Prod. Res. 2008, 22, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Bannon, C.D.; Craske, J.D.; Ngo, T.H.; Harper., N.L.; O’rourke, K.L. Analysis of fatty acid methyl esters with high accuracy and reliability. J. Chromatogr. 1982, 247, 63–69. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Besbes Hlila, M.; Mosbahc, H.; Mssadad, K.; Ben Jannet, H.; Aouni, M.; Boulbaba, S. Acetylcholinesterase inhibitory and antioxidant properties of roots extracts from the Tunisian Scabiosa arenaria Forssk. Ind. Crop. Prod. 2015, 67, 62–69. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Papamaloni, E.; Tzanetakis, N.; Tzanetaki, E.L.; Kotzekidou, P. Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci. 2003, 65, 859–867. [Google Scholar]
- Sample Availability: Samples of the compounds 1 and 2 are available in small amounts from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salah, N.B.; Casabianca, H.; Jannet, H.B.; Chenavas, S.; Sanglar, C.; Fildier, A.; Bouzouita, N. Phytochemical and Biological Investigation of Two Diplotaxis Species Growing in Tunisia: D. virgata & D. erucoides. Molecules 2015, 20, 18128-18143. https://doi.org/10.3390/molecules201018128
Salah NB, Casabianca H, Jannet HB, Chenavas S, Sanglar C, Fildier A, Bouzouita N. Phytochemical and Biological Investigation of Two Diplotaxis Species Growing in Tunisia: D. virgata & D. erucoides. Molecules. 2015; 20(10):18128-18143. https://doi.org/10.3390/molecules201018128
Chicago/Turabian StyleSalah, Nizar Ben, Hervé Casabianca, Hichem Ben Jannet, Sophie Chenavas, Corinne Sanglar, Aurélie Fildier, and Nabiha Bouzouita. 2015. "Phytochemical and Biological Investigation of Two Diplotaxis Species Growing in Tunisia: D. virgata & D. erucoides" Molecules 20, no. 10: 18128-18143. https://doi.org/10.3390/molecules201018128
APA StyleSalah, N. B., Casabianca, H., Jannet, H. B., Chenavas, S., Sanglar, C., Fildier, A., & Bouzouita, N. (2015). Phytochemical and Biological Investigation of Two Diplotaxis Species Growing in Tunisia: D. virgata & D. erucoides. Molecules, 20(10), 18128-18143. https://doi.org/10.3390/molecules201018128