2.1. Chemical Compositions of EOs
Extracted by the steam distillation method, the yield ratios (%) of Angelica oil, Chuanxiong oil, Cyperus oil, Cinnamon oil and Clove oil were determined to be 0.39% ± 0.08%, 0.61% ± 0.13%, 1.20% ± 0.06%, 3.57% ± 0.40% and 13.80% ± 0.31% (n = 3), respectively. The constituents of the five EOs (Angelica oil, Chuanxiong oil, Cyperus oil, Cinnamon oil and Clove oil) identified by GC-MS are shown in
Table 1,
Table 2,
Table 3,
Table 4 and
Table 5, respectively. The major components of Angelica oil, Chuanxiong oil, Cinnamon oil and Clove oil were ligustilide (78.44%), ligustilide (41.28%),
trans-cinnamaldehyde (82.75%) and eugenol (80.47%), respectively. For Cyperus oil, the main components were cyperene (27.69%), dehydrofukinone (27.54%) and α-cyperone (7.94%).
Table 1.
Chemical composition of Angelica oil.
Table 1.
Chemical composition of Angelica oil.
| Peak No. | Retention Time (min) | Identified Compound | CAS | Relative Content (%) |
|---|
| 1 | 6.252 | (1S)-(−)-α-Pinene | 7785-26-4 | 0.81 |
| 2 | 9.602 | trans-β-Ocimene | 3779-61-1 | 4.97 |
| 3 | 12.724 | (4E,6Z)-2,6-Dimethyl-2,4,6-octatriene | 7216-56-0 | 0.13 |
| 4 | 13.680 | 6-Butyl-1,4-cycloheptadiene | 22735-58-6 | 0.94 |
| 5 | 17.629 | 6-Undecanone | 927-49-1 | 0.16 |
| 6 | 18.985 | 2-Methoxy-4-vinylphenol | 7786-61-0 | 0.39 |
| 7 | 20.168 | 2,4,5-Trimethylbenzaldehyde | 5779-72-6 | 0.55 |
| 8 | 20.287 | Eugenol | 97-53-0 | 0.69 |
| 9 | 20.767 | α-Cubebene | 17699-14-8 | 0.16 |
| 10 | 21.821 | 2-Isopropyl-5-methyl-9-methylene[4.4.0]dec-1-ene | 150320-52-8 | 0.38 |
| 11 | 22.599 | 3-Methylenecycloheptene | 34564-56-2 | 0.50 |
| 12 | 23.020 | Acoradiene | 24048-44-0 | 0.27 |
| 13 | 23.555 | Chamigrene | 18431-82-8 | 0.20 |
| 14 | 24.111 | 1,5,5-Trimethyl-6-methylenecyclohexene | 514-95-4 | 0.70 |
| 15 | 24.376 | (+)-Cuparene | 16982-00-6 | 0.13 |
| 16 | 24.473 | β-Bisabolene | 495-61-4 | 0.27 |
| 17 | 25.138 | (+)-β-Himachalene | 1461-03-6 | 0.17 |
| 18 | 26.553 | Espatulenol | 6750-60-3 | 1.12 |
| 19 | 29.049 | 2-Chloro-1-(2,4-dimethylphenyl)-2-methyl-1-Propanone | 54965-53-6 | 1.28 |
| 20 | 29.762 | Butylidenephthalide | 551-08-6 | 4.13 |
| 21 | 29.983 | N,N'-Diacetyl-1,4-phenylenediamine | 140-50-1 | 0.24 |
| 22 | 31.377 | trans-3n-Butylidenephthalide | 1000365-98-3 | 0.76 |
| 23 | 31.582 | 2-Propyl-phenol | 644-35-9 | 0.63 |
| 24 | 32.290 | Ligustilide | 4431-01-0 | 78.44 |
| 25 | 34.348 | trans-Ligustilide | 1000365-98-8 | 1.29 |
Table 2.
Chemical composition of Chuanxiong oil.
Table 2.
Chemical composition of Chuanxiong oil.
| Peak No. | Retention Time (min) | Identified Compound | CAS | Relative Content (%) |
|---|
| 1 | 3.031 | (+)-α-Pinene | 7785-70-8 | 1.01 |
| 2 | 3.651 | Sabenene | 3387-41-5 | 0.69 |
| 3 | 4.518 | α-Terpinen | 99-86-5 | 0.87 |
| 4 | 4.684 | p-Cymene | 99-87-6 | 1.54 |
| 5 | 5.390 | γ-Terpinene | 99-85-4 | 2.69 |
| 6 | 5.999 | Terpinolene | 586-62-9 | 2.31 |
| 7 | 7.342 | 1(2H)-Naphthalenone,3,4,4a,5,8,8a-hexahydro- | 14116-78-0 | 3.05 |
| 8 | 7.722 | l-Terpinen-4-ol | 20126-76-5 | 3.99 |
| 9 | 10.230 | 2-Methoxy-4-vinylphenol | 7786-61-0 | 0.68 |
| 10 | 11.134 | 1-Phenyl-1-pentanone | 1009-14-9 | 1.03 |
| 11 | 12.905 | β-curcumene | 1000374-17-4 | 2.04 |
| 12 | 14.702 | β-Eudesmene | 17066-67-0 | 6.16 |
| 13 | 14.974 | α-Selinene | 473-13-2 | 1.99 |
| 14 | 17.734 | Espatulenol | 6750-60-3 | 1.51 |
| 15 | 20.532 | Methyl 4-ethylbenzoate | 7364-20-7 | 4.95 |
| 16 | 21.334 | Butylidenephthalide | 551-08-6 | 7.12 |
| 17 | 21.580 | p-Phenylenediacetamide | 140-50-1 | 1.99 |
| 18 | 22.099 | trans-Galbanolene | 19883-29-5 | 1.28 |
| 19 | 23.324 | Fenipentol | 583-03-9 | 10.09 |
| 20 | 23.522 | 1,2,3,5,6,7-Hexahydro-inden-4-one | 22118-01-0 | 3.74 |
| 21 | 24.003 | Ligustilide | 4431-01-0 | 41.28 |
Table 3.
Chemical composition of Cyperus oil.
Table 3.
Chemical composition of Cyperus oil.
| Peak No. | Retention Time (min) | Identified Compound | CAS | Relative Content (%) |
|---|
| 1 | 4.479 | trans-(−)-Pinocarveol | 547-61-5 | 0.77 |
| 2 | 5.565 | (−)-Myrtenol | 19894-97-4 | 0.97 |
| 3 | 9.067 | B-Damascone | 35044-68-9 | 0.97 |
| 4 | 10.640 | 7-(1,1-Dimethylethyl)-3,4-dihydro-1(2H)-naphthalenone | 22583-68-2 | 1.59 |
| 5 | 11.245 | α-Cubebene | 17699-14-8 | 1.08 |
| 6 | 12.337 | Cyperene | 2387-78-2 | 27.69 |
| 7 | 15.287 | Rotundene | 1000374-17-0 | 3.52 |
| 8 | 16.937 | β-Eudesmene | 17066-67-0 | 3.04 |
| 9 | 19.774 | 4-(4-Methoxyphenyl)iminopentan-2-one | 20010-38-2 | 5.94 |
| 10 | 24.629 | Caryophyllene oxide | 1139-30-6 | 7.90 |
| 11 | 28.641 | 9H-Cycloisolongifolene, 8-oxo- | 1000155-43-0 | 3.83 |
| 12 | 37.046 | (−)-Isolongifolen-9-one | 26839-52-1 | 3.95 |
| 13 | 38.874 | Dehydrofukinone | 19598-45-9 | 27.54 |
| 14 | 45.599 | 1,2,3,4,5,6-Hexahydro-1,1,5,5-tetramethyl-7H-2,4a-methanonaphthalen-7-one | 23747-14-0 | 3.26 |
| 15 | 46.987 | α-Cyperone | 473-08-5 | 7.94 |
Table 4.
Chemical composition of Cinnamon oil.
Table 4.
Chemical composition of Cinnamon oil.
| Peak No. | Retention Time (min) | Identified Compound | CAS | Relative Content (%) |
|---|
| 1 | 4.239 | Benzenepropanal | 104-53-0 | 0.22 |
| 2 | 4.323 | Borneol | 507-70-0 | 0.10 |
| 3 | 5.157 | 3-Phenyl-2-propenal | 104-55-2 | 0.47 |
| 4 | 6.249 | trans-Cinnamaldehyde | 14371-10-9 | 82.75 |
| 5 | 8.763 | (+)-Cyclosativene | 22469-52-9 | 0.24 |
| 6 | 9.078 | α-Cubebene | 17699-14-8 | 5.93 |
| 7 | 9.778 | (+)-Sativene | 3650-28-0 | 0.22 |
| 8 | 10.604 | 1-Caryophyllene | 87-44-5 | 0.15 |
| 9 | 12.789 | γ-Muurolene | 30021-74-0 | 0.59 |
| 10 | 13.475 | γ-Maaliene | 20071-49-2 | 0.19 |
| 11 | 13.636 | α-Muurolene | 31983-22-9 | 2.16 |
| 12 | 14.000 | 1,2,4a,5,6,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)naphthalene | 483-75-0 | 0.17 |
| 13 | 14.224 | d-Cadinene | 483-76-1 | 3.61 |
| 14 | 14.441 | 1,6-Dimethyl-4-(1-methylethyl)-(1,2,3,4,4a,7)Hexahydronaphthalene | 16728-99-7 | 1.37 |
| 15 | 14.714 | 1,1,6-Trimethyl-1,2-dihydronaphthalene | 30364-38-6 | 0.25 |
| 10 | 17.746 | t-Muurolol | 19912-62-0 | 0.69 |
| 11 | 17.9 | α-Copaene | 3856-25-5 | 0.38 |
Table 5.
Chemical composition of Clove oil.
Table 5.
Chemical composition of Clove oil.
| Peak No. | Retention Time (min) | Identified Compound | CAS | Relative Content (%) |
|---|
| 1 | 8.783 | Eugenol | 97-53-0 | 80.47 |
| 2 | 10.024 | Caryophyllene | 87-44-5 | 1.72 |
| 3 | 10.719 | Humulene | 6753-98-6 | 0.23 |
| 4 | 12.164 | Eugenyl acetate | 93-28-7 | 17.43 |
| 5 | 13.297 | Caryophyllene oxide | 1139-30-6 | 0.15 |
2.3. In vitro Skin Permeation
Although the pig’s ear skin was considered to be the best model, due to the convenience of
in vitro and
in vivo comparison, rat skin was usually applied as skin model to investigate the skin permeation behavior [
9,
13,
20]. The effect of EOs on the
in vitro skin permeation profiles of ibuprofen through excised rat skin is shown in
Figure 2. The permeation kinetics within 18 h followed zero order kinetics. The skin permeation parameters of ibuprofen with different EOs are listed in
Table 6. All EOs significantly enhanced both the steady state flux and permeation ratio of ibuprofen compared to the control (
p < 0.001). Among the EOs, the highest permeation rate of 52.05 ± 7.83 µg/cm
2/h was obtained with Chuanxiong oil, while Cyperus oil produced the lowest enhancement in ibuprofen flux (32.97 ± 11.72 µg/cm
2/h). Furthermore, except Cyperus oil, the ER values of EOs were all higher than that of Azone, especially Chuanxiong oil.
Figure 2.
Skin permeation profiles of ibuprofen with various PEs through excised rat skin (n = 5). Control: ibuprofen hydrogel without PE.
Figure 2.
Skin permeation profiles of ibuprofen with various PEs through excised rat skin (n = 5). Control: ibuprofen hydrogel without PE.
Table 6.
Effect of PEs on the percutaneous permeation parameters of ibuprofen through excised rat skin (n = 5)
Table 6.
Effect of PEs on the percutaneous permeation parameters of ibuprofen through excised rat skin (n = 5)
| PE | Flux/µg·cm-2·h-1 | ER | Q48/µg·cm-2 | Permeation Ratio a/% |
|---|
| Angelica oil | 48.88 ± 8.92 *** | 3.35 | 1102.57 ± 94.58 *** | 57.70 |
| Chuanxiong oil | 52.05 ± 7.83 ***,# | 3.57 | 1285.15 ± 109.92 ***,# # | 67.26 |
| Cyperus oil | 32.97 ± 11.72 *** | 2.26 | 790.28 ± 118.71 *** | 41.35 |
| Cinnamon oil | 40.78 ± 9.99 *** | 2.80 | 1084.21 ± 162.80 *** | 56.74 |
| Clove oil | 44.70 ± 7.89 *** | 3.07 | 1201.02 ± 197.02 ***,# | 62.85 |
| Azone | 37.10 ± 8.51 *** | 2.55 | 912.33 ± 178.13 *** | 47.74 |
| Control | 14.57 ± 3.47 | - | 404.29 ± 106.10 | 21.16 |
Among NSAID drugs, it was found that ibuprofen showed the poorest bioavailability. Following transdermal administration to rats, the area under the plasma–time curve (AUC) values of piroxicam, ketoprofen, naproxen, indomethacin and ibuprofen were determined to be 527.00, 269.45, 258.64, 243.22 and 88.08 μg/mL·h, respectively [
13]. Therefore, it is indispensable to employ PE to increase the skin permeation of ibuprofen in order to maintain an effective blood level. Among the five EOs, only Clove oil had been evaluated as PE for TDD of ibuprofen hydrogel and the AUC values was increased by 2.4 times compared to the control [
21]. In the present study, five analgesic EOs were compared as PE. Compared to the control (ibuprofen only), the ER values of ibuprofen
in vitro were measured to be 3.35, 3.57, 2.26, 2.80, 3.07 and 2.55 using Angelica oil, Chuanxiong oil, Cyperus oil, Cinnamon oil, Clove oil and Azone as PE, respectively. As PE, EOs act mainly by disruption of the highly ordered structure of SC lipid with an increase in intercellular diffusivity [
22]. And the intercellular spaces are the preferential pathway for lipophilic ibuprofen to penetrate through the SC.
2.4. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR) Spectroscopic Studies
To elucidate the effect of EOs on the intercellular lipids in the SC, ATR-FTIR stretching peaks near 2850 cm
-1 (C-H symmetric stretching absorbance frequency peak), 2920 cm
−1 (C-H asymmetric stretching absorbance frequency peak), 1650 (Amide I) and 1540 (Amide II) were measured after the application of EOs to skin (
Figure 3). The shift of a higher frequency occurs when the CH
2 groups along the alkyl chain of lipids change from
trans to
gauche conformation, suggesting that the SC lipid is disturbed [
23]. Peaks near 2920 cm
−1 and 2850 cm
−1, for asymmetric and symmetric stretching, respectively, showed that treatment of rat skin with the solvent (PG:IPA = 30:70 (
v/
v)) alone did not result in an obvious shift in stretching frequencies compared with untreated skin. In contrast, in skin treated with EOs or Azone, shifts were observed to CH
2 symmetric and asymmetric stretching frequencies which were approximately 0.4 to 6.8 cm
−1 higher than those observed with no treatment (blank) or with solvent alone (control). The peaks obtained near 2850 and 2920 cm
−1 correspond to asymmetric and symmetric stretching modes, respectively, of the terminal methylene groups of the lipids, and these provided specific information about the interior composition of the lipid bilayer. The
trans/
gauche ratio of the alkyl tails affect the frequencies of the FTIR bands. A change in the band position from
trans to
gauche conformation indicates fluidization of the lipid bilayer. The magnitude of blue shift in the peak position of the asymmetric and symmetric stretching vibration absorbance is correlated with an increase number of
gauche conformers in the lipid acyl chain. The higher the shifts, the higher the ratio of
gauche to
trans [
24]. In summary, the results of ATR-FTIR studies revealed that EOs acted mainly by disruption of the highly ordered structure of SC lipid with an increase in intercellular diffusivity, which helped lipophilic ibuprofen to penetrate through the SC. In addition, according to the change in peak position of amide I or II, the five EOs also appeared to have a little effect on keratin, especially Chuanxiong oil and Angelica oil.
Figure 3.
ATR-FTIR absorption spectra of rat skin treated with various PEs for 12 h. Note: the peak position values are the average of three replicates.
Figure 3.
ATR-FTIR absorption spectra of rat skin treated with various PEs for 12 h. Note: the peak position values are the average of three replicates.
2.5. Pharmacological Experiments
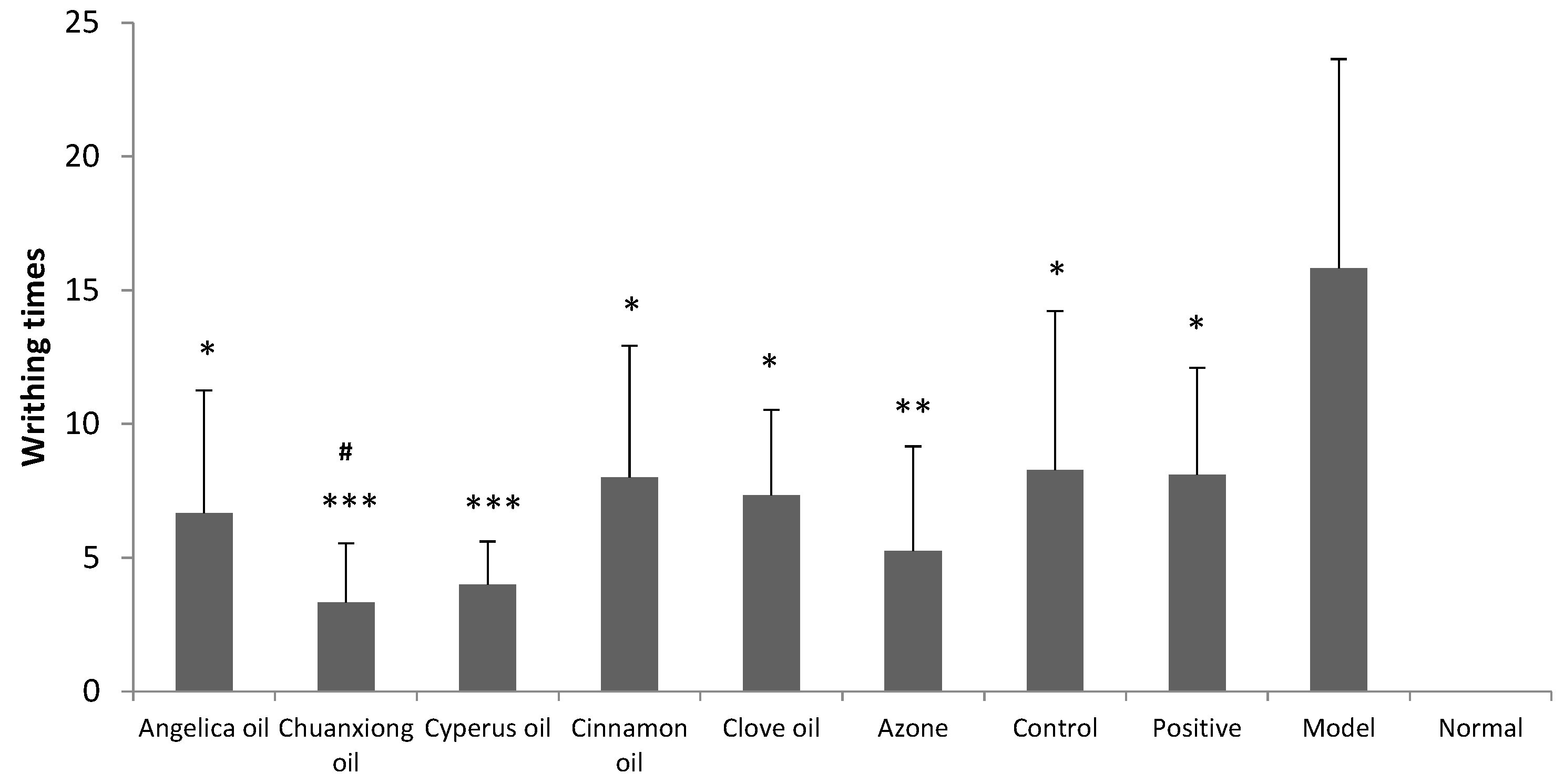
Results obtained from writhing test in dysmenorrheal model mice are shown in
Figure 4. Model group and positive group were treated with blank hydrogel (transdermal administration) and the ibuprofen suspension (oral administration), respectively. Positive group, control group (transdermal administration of ibuprofen hydrogel without PE) and ibuprofen hydrogel containing different EO groups all produced significant decrease in the number of abdominal writhing response at the dose of 140 mg/kg/day (
p < 0.05). The pain inhibitory intensity of ibuprofen hydrogel was obviously increased with Chuanxiong oil compared to the control (
p < 0.05).
Figure 4.
Inhibitory effects of ibuprofen (140 mg/kg/d) with different EOs on dysmenorrheal model mice’s writhing times (n = 8). Positive: oral administration of 56 mg/kg/d ibuprofen suspension. Control: transdermal administration of ibuprofen hydrogel without PE. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the model group. # p < 0.05 vs. the control group.
Figure 4.
Inhibitory effects of ibuprofen (140 mg/kg/d) with different EOs on dysmenorrheal model mice’s writhing times (n = 8). Positive: oral administration of 56 mg/kg/d ibuprofen suspension. Control: transdermal administration of ibuprofen hydrogel without PE. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the model group. # p < 0.05 vs. the control group.
As shown in
Figure 5, in primary dysmenorrheal model mice, the contents of Ca
2+ and PGF
2α in uterus tissue homogenate were significantly increased (
p < 0.05) and the level of NO was remarkably decreased (
p < 0.05). The addition of EOs, especially Chuanxiong oil, could reduce the contents of Ca
2+ and PGF
2α and increase the level of NO in uterus tissue homogenate.
Figure 5.
Effects of ibuprofen (140 mg/kg/day) with different EOs on the levels of calcium ion, nitric oxide and PGF2α in uterine tissue of dysmenorrheal model mice (n = 8). Positive: oral administration of 56 mg/kg/day ibuprofen suspension. Control: transdermal administration of ibuprofen hydrogel without PE. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the model group. # p < 0.05, ## p < 0.05, ### p < 0.05 vs. the control group (ibuprofen only).
Figure 5.
Effects of ibuprofen (140 mg/kg/day) with different EOs on the levels of calcium ion, nitric oxide and PGF2α in uterine tissue of dysmenorrheal model mice (n = 8). Positive: oral administration of 56 mg/kg/day ibuprofen suspension. Control: transdermal administration of ibuprofen hydrogel without PE. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. the model group. # p < 0.05, ## p < 0.05, ### p < 0.05 vs. the control group (ibuprofen only).
Ibuprofen belongs to the NSAID family and exerts its main pharmacodynamic actions through inhibition of two isoforms of cyclooxygenase, COX-1 and COX-2. The analgesic, antipyretic, and anti-inflammatory activity of NSAIDs appears to operate mainly through inhibition of COX-2, whereas inhibition of COX-1 would be responsible for unwanted effects on the gastrointestinal tract [
25]. COX enzymes are involved in inflammatory pathways and responsible for formation of pro-inflammatory prostaglandins. Prostaglandins which can cause constriction in uterine smooth muscle and sensitize spinal neurons to pain are regarded as the most pain factor to primary dysmenorrohea [
26]. PGF
2α, a naturally occurring prostaglandin, is used to induce labor in medicine. PGF
2α combined with its receptor on the spiral arterioles increased uterine contractility, resulting in ischemia pain [
26]. In the present study, it was found that the PGF
2α levels in uterus were all significantly decreased after treatment with ibuprofen or ibuprofen with EO groups, especially for Chuanxiong oil groups which showed the highest enhancement effects in skin permeation studies of ibuprofen.
The levels of Ca
2+ and NO in the dysmenorrheal model mice uterus were also detected. Overload of intracellular Ca
2+ can cause uterine smooth muscle contraction and reduce the endometrial blood supply, leading to the occurrence of dysmenorrhoea. Therefore, calcium channel blocking agents decrease myometrial contractility and beneficial in cases of dysmenorrhea [
27]. The treatment of ibuprofen, whether by oral or TDD route, all resulted in the significant decrease of Ca
2+ levels, especially in combination with Chuanxiong oil. This indicated that one mechanism of ibuprofen treating dysmenorrhea may act on Ca
2+ channel to decrease intracellular Ca
2+ concentration. NO could regulate uterine contraction and execute pain modulation of peripheral and central levels [
28]. As NO was negatively correlated with pain in acute inflammation [
29], the significant increase of NO levels in uterus, which was observed in miceof Chuanxiong oil, Clove oil or Azone groups, contributed to inhibit analgesia. In summary, by comparing the writhing times and levels of PGF
2α, Ca
2+ and NO in uterus, TDD of ibuprofen in combination with Chuanxiong oil was found to be the most effective in the treatment of dysmenorrhea.
Rhizoma Chuanxiong, known as chuanxiong in China, is one of the most frequently used drugs in the prescriptions of traditional Chinese medicine for treating cardiovascular diseases, menstrual disorders and gynecological problems. Chuanxiong oil, rich in phthalide components, is generally claimed to play the major role in the heaemodynamic and analgesic effects of the herb. It had been demonstrated that Chuanxiong oil possessed potent analgesic and sedative activity [
30,
31] and was effective in relaxing vessels [
32]. In addition, Chuanxiong oil had been proved to be a satisfactory PE for TDD of flurbiprofen [
33], a lipophilic drug similar to ibuprofen.
It was noteworthy that a discrepancy between the percutaneous absorption and pharmacological studies, especially for Cyperus oil. The analgesic efficacy of Cyperus oil [
19] seemed to be much higher than its permeation enhancement effect.