Analysis of Chemical Constituents in Wuzi-Yanzong-Wan by UPLC-ESI-LTQ-Orbitrap-MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Analytical Conditions
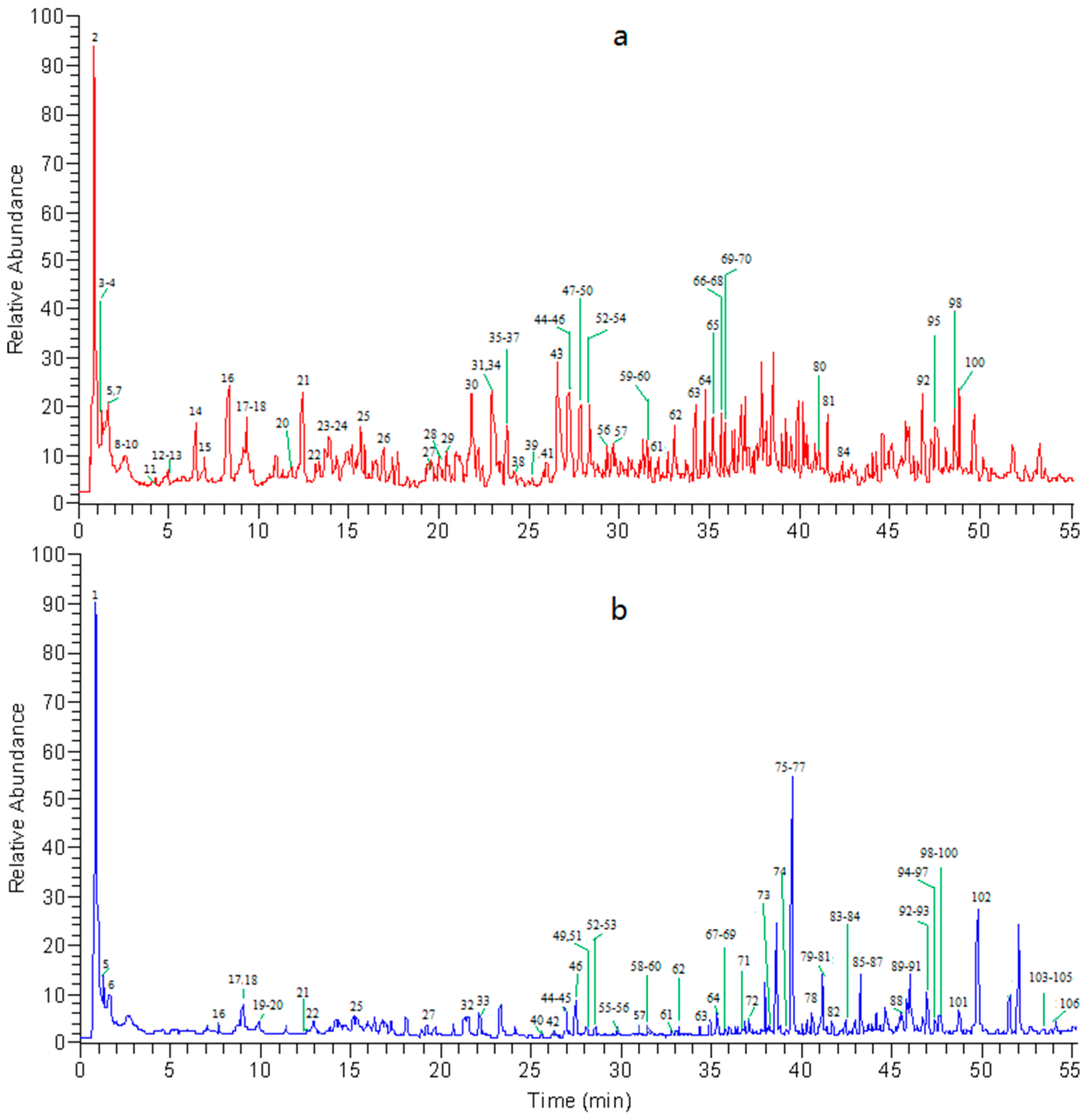
2.2. Chemical Constituents Identification in WZYZW
| No. | tR (min) | Molecular Formula | Caculated Mass (m/z) | Experimental Mass (m/z) | MS/MS Fragments | Identification Compound | Source | |||
|---|---|---|---|---|---|---|---|---|---|---|
| N-ion | ppm | P-ion | ppm | |||||||
| 1 | 0.81 | C5H11NO2 | 117.08626 | ― | ― | 118.08569 | −0.6 | 235[2M + H]+; | Abromine | a |
| 118[M + H]+; | ||||||||||
| 59[C3H9N]+; | ||||||||||
| 58[C3H8N]+ | ||||||||||
| 2 | 0.87 | C6H8O6 | 176.02371 | 175.02289 | −4.7 | ― | ― | 175[M − H]−; | Ascorbic acid | a |
| 115[C4H3O4]−; | ||||||||||
| 113[C5H5O3]− | ||||||||||
| 3 | 1.12 | C6H8O7 | 192.01863 | 191.0189 | 1.4 | ― | ― | 191[M − H]−; | Citric acid | d |
| 173[M − H − H2O]−; | ||||||||||
| 129[M − H − H2O − CO2]−; | ||||||||||
| 111[M − H − H2O − COOH − OH]− | ||||||||||
| 4 | 1.12 | C4H6O5 | 134.01315 | 133.01372 | 4.3 | ― | ― | 133[M + H]+; | Malic acid | d |
| 115[M + H − H2O]+ | ||||||||||
| 5 | 1.28 | C6H5NO2 | 123.03931 | 122.02417 | 4.2 | 124.03876 | −4.3 | 122[M − H]−; | Nicotinic acid | a |
| 124[M + H]+; | ||||||||||
| 106 [M + H − H2O]+ | ||||||||||
| 6 | 1.58 | C12H16N4OS | 264.11176 | ― | ― | 265.11071 | −3.9 | 265[M + H]+; | Thiamine | a |
| 156[C7H10NOS]+; | ||||||||||
| 144[C6H10NOS]+; | ||||||||||
| 122[C6H8N3]+ | ||||||||||
| 7 | 1.97 | C4H6O4 | 118.01824 | 117.01791 | −2.8 | ― | ― | 117[M − H]−; | Succinic acid | d |
| 99[M − H − H2O]−; | ||||||||||
| 73[M − H − CO2]− | ||||||||||
| 8 | 2.68 | C7H6O5 | 170.01315 | 169.01301 | −0.8 | ― | ― | 169[M − H]−; | Gallic acid | c |
| 125[M – H − CO2]− | ||||||||||
| 9 | 3.08 | C4H6O6 | 150.00806 | 149.00808 | 0.1 | ― | ― | 149[M − H]−; | Tartaric acid | d |
| 131[M − H − H2O]−; | ||||||||||
| 103[M − H − HCOOH]− | ||||||||||
| 10 | 3.28 | C17H20N4O6 | 376.12991 | 375.12988 | −0.1 | ― | ― | 375[M − H]−; | Riboflavin | a |
| 255[C13H11N4O2]−; | ||||||||||
| 212[C12H10N3O]− | ||||||||||
| 11 | 4.48 | C7H6O3 | 138.02332 | 137.02365 | 2.4 | ― | ― | 137[M − H]−; | Salicylic acid/ | c |
| 93[C6H5O]− | 4-Hydroxyben-zoic acid | |||||||||
| 12 | 4.66 | C7H6O4 | 154.01824 | 153.01862 | 2.5 | ― | ― | 153[M − H]−; | Protocatechuic acid | d |
| 109[M − H − CO2]− | ||||||||||
| 13 | 4.74 | C7H6O3 | 138.02332 | 137.02373 | 3 | ― | ― | 137[M − H]−; | Salicylic acid/ | c |
| 93[C6H5O]− | 4-Hydroxyben-zoic acid | |||||||||
| 14 | 7.2 | C2H7NO3S | 125.00629 | 124.00638 | 0.7 | ― | ― | 124[M − H]−; | Taurine | a |
| 80[SO3]− | ||||||||||
| 15 | 7.38 | C8H8O4 | 168.03389 | 167.0342 | 1.9 | ― | ― | 167[M − H]−; | Vanillic acid | a,c |
| 152[M − H − CH3]−; | ||||||||||
| 123[M − H − CO2]−; | ||||||||||
| 108[M − H − CH3 − CO2]− | ||||||||||
| 16 | 8.35 | C16H22O10 | 374.11293 | 373.11377 | 2.3 | 397.10892 | −4.0 | 373[M − H]−; | Geniposidic acid | e |
| 329[M − H − CO2]−; | ||||||||||
| 211[M − H − Glc]−; | ||||||||||
| 193 [M − H − Glc − H2O]−; | ||||||||||
| 167[M − H − Glc − CO2]−; | ||||||||||
| 149[M − H − Glc − CO2 − H2O]−; | ||||||||||
| 397[M + Na]+; | ||||||||||
| 353[M + Na − CO2]+; | ||||||||||
| 235[M + Na − Glc]+; | ||||||||||
| 217[M + Na − Glc − H2O]+; | ||||||||||
| 149[C7H10O2Na]+ | ||||||||||
| 17 | 8.79 | C7H12O6 | 192.05502 | 191.05503 | 0.1 | 193.06981 | −4.4 | 191[M − H]−; | Quinic acid | d |
| 173[M − H − H2O]−; | ||||||||||
| 155[M − H − 2H2O]−; | ||||||||||
| 127[M − H − 2H2O − CO]−; | ||||||||||
| 193 [M + H]+; | ||||||||||
| 175[M + H − H2O]+; | ||||||||||
| 157[M + H − 2H2O]+ | ||||||||||
| 18 | 9.68 | C9H8O4 | 180.03389 | 179.03429 | 2.2 | 181.04889 | −3.5 | 179[M − H]−; | Caffeic acid | a |
| 135[M − H − CO2]−; | ||||||||||
| 181[M + H]+; | ||||||||||
| 163[M + H − H2O]+; | ||||||||||
| 145[M + H − 2H2O]+ | ||||||||||
| 19 | 10.2 | C17H23NO3 | 289.17507 | ― | ― | 290.17578 | 2.4 | 290[M + H]+; | Atropine | a |
| 272[M + H − H2O]+; | ||||||||||
| 260[M + H − HCHO]+; | ||||||||||
| 242[M + H − HCHO − H2O]+; | ||||||||||
| 124[C8H14N]+; | ||||||||||
| 95[C7H11]+ | ||||||||||
| 20 | 11.91 | C10H10O4 | 194.04954 | 193.05001 | 2.4 | 195.06447 | −3.7 | 193[M − H]−; | Ferulic Acid | a,c |
| 175[M − H − H2O]−; | ||||||||||
| 149[M − H − CO2]−; | ||||||||||
| 134[M − H − CH3 − CO2]−; | ||||||||||
| 195[M + H]+; | ||||||||||
| 177[M + H − H2O]+; | ||||||||||
| 145[M + H − H2O − CH3 − OH]+ | ||||||||||
| 21 | 12.37 | C16H18O9 | 354.08671 | 353.08731 | 1.7 | 355.10097 | −3.9 | 353[M − H]−; | Chlorogenic acid | a |
| 191[C7H11O6]−; | ||||||||||
| 173[C7H9O5]−; | ||||||||||
| 127[C6H7O3]−; | ||||||||||
| 355[M + H]+; | ||||||||||
| 163[C9H7O3]+; | ||||||||||
| 117[C8H5O]+ | ||||||||||
| 22 | 13.1 | C9H8O3 | 164.03897 | 163.03905 | 0.5 | 165.05393 | −4.2 | 163[M − H]−; | p-Coumaric acid | a |
| 119[M − H − CO2]−; | ||||||||||
| 165 [M + H]+; | ||||||||||
| 147[M + H − H2O]+ | ||||||||||
| 23 | 13.64 | C7H10O5 | 174.04445 | 173.04381 | −3.7 | ― | ― | 173[M − H]−; | Shikimic acid | c |
| 155[M − H − H2O]−; | ||||||||||
| 137[M − H − 2H2O]−; | ||||||||||
| 128[M − H − COOH]− | ||||||||||
| 24 | 13.97 | C27H30O17 | 626.24908 | 625.25148 | 3.8 | ― | ― | 625[M − H]−; | Quercetin-3-O-β-d-galactopyra-noside-7-O-β-d-glucopyranoside | b |
| 463[M − H − Gal]−; | ||||||||||
| 301[M − H − Gal − Glc]−; | ||||||||||
| 300[M − H − Gal − Glc − H]−; | ||||||||||
| 273[M − H − Gal − Glc − CO]−; | ||||||||||
| 255[M − H − Gal − Glc − CO − H2O]− | ||||||||||
| 25 | 15.6 | C9H6O4 | 178.01824 | 177.01847 | 1.3 | 179.03317 | −4.0 | 177[M − H]−; | Esculetin | c |
| 149[M − H − CO]−; | ||||||||||
| 133[M − H − CO2]−; | ||||||||||
| 121[M − H − 2CO]−; | ||||||||||
| 105[M − H − CO2 − CO]−; | ||||||||||
| 93[M − H − 3CO]−; | ||||||||||
| 179[M + H]+; | ||||||||||
| 151[M + H − CO]+; | ||||||||||
| 133[M + H − CO − H2O]+ | ||||||||||
| 26 | 17.17 | C22H22O11 | 462.10784 | 461.10962 | 3.9 | ― | ― | 461[M − H]−; | Homoplantagini-n | e |
| 299[M − H − Glu]−; | ||||||||||
| 271[M − H − Glu − CO]−; | ||||||||||
| 181[C8H5O5]− | ||||||||||
| 27 | 19.43 | C10H8O4 | 192.03387 | 191.03419 | 1.6 | 193.04933 | −1.1 | 191[M − H]−; | Scopoletin | a |
| 193[M + H]+; | ||||||||||
| 178[M + H − CH3]+; | ||||||||||
| 165[M + H − CO]+; | ||||||||||
| 161[M + H − CH3OH]+; | ||||||||||
| 133[M + H − CH3OH − CO]+ | ||||||||||
| 28 | 19.6 | C21H20O12 | 464.08711 | 463.08829 | 2.6 | ― | ― | 463[M − H]−; | 6-Hydroxy-luteolin-7-O-glucoside | e |
| 301[M − H − Glc]−; | ||||||||||
| 273[M − H − Glc − CO]−; | ||||||||||
| 257[M − H − Glc − CO2]−; | ||||||||||
| 167[C7H3O5]− | ||||||||||
| 29 | 20.93 | C22H20O12 | 476.0871 | 475.08633 | −1.6 | ― | ― | 475[M − H]−; | Kaempferol-3-O-β-d-glucuronic acid methyl ester | c |
| 457[M − H − H2O]−; | ||||||||||
| 433[M − H − CO=CH2]−; | ||||||||||
| 415[M − H − CH3COOH]−; | ||||||||||
| 285[M − H − C7H10O6]− | ||||||||||
| 30 | 21.62 | C15H10O7 | 302.03428 | 301.03396 | −1.1 | ― | ― | 301[M − H]−; | 6-Hydroxy-luteolin | e |
| 273[M − H − CO]−; | ||||||||||
| 257[M − H − CO2]−; | ||||||||||
| 167[C7H3O5]− | ||||||||||
| 31 | 21,78 | C26H28O16 | 596.14463 | 595.14543 | 1.4 | ― | ― | 595[M − H]−; | Quercetin-3-O-β-d-galactosyl-(1→2)-β-d-apioside | b |
| 505[M − H – Api + C2H2O]−; | ||||||||||
| 463[M − H − Api]−; | ||||||||||
| 445[M − H − Api − H2O]−; | ||||||||||
| 301[M − H − Api − Gal]−; | ||||||||||
| 300[M − H − Api − Gal − H]−; | ||||||||||
| 273[M − H − Api − Gal − CO]−; | ||||||||||
| 257[M − H − Api − Gal − 2CO]−; | ||||||||||
| 179[C8H3O5]− | ||||||||||
| 32 | 21.88 | C14H6O8 | 302.01355 | ― | ― | 303.01321 | −1.1 | 303[M+H]+; | Ellagic acid | c |
| 285[M + H − H2O]+; | ||||||||||
| 275[M + H − CO]+; | ||||||||||
| 257[M + H − CO − H2O]+; | ||||||||||
| 247[M + H − 2CO]+; | ||||||||||
| 201[C11H5O4]+ | ||||||||||
| 33 | 22.26 | C29H28O9 | 520.18061 | ― | ― | 521.18042 | −0.4 | 521[M + H]+; | Schisantherin D | d |
| 399[M + H − C6H5COOH]+; | ||||||||||
| 355[M + H − C6H5COOH − C2H4O]+ | ||||||||||
| 34 | 22.9 | C21H20O12 | 464.08711 | 463.08887 | 3.8 | ― | ― | 463[M − H]−; | Hyperoside | b,c |
| 301[M − H − Gal]−; | ||||||||||
| 255[M − H − Gal − CO − H2O]−; | ||||||||||
| 151[C7H3O4]−; | ||||||||||
| 107[C6H3O2]− | ||||||||||
| 35 | 23.02 | C16H12O6 | 300.05502 | 299.05453 | −1.6 | ― | ― | 299[M − H]−; | Hispidulin | e |
| 271[M − H − CO]−; | ||||||||||
| 255[M − H − CO2]−; | ||||||||||
| 227[M − H − CO − CO2]−; | ||||||||||
| 181[C8H5O5]− | ||||||||||
| 36 | 23.1 | C27H30O16 | 610.14501 | 609.1479 | 4.7 | ― | ― | 609[M − H]−; | Rutin | c |
| 343[C17H11O8]−; | ||||||||||
| 301[M − H − Rha − Glc]−; | ||||||||||
| 300[M − H − Rha − Glc − H]−; | ||||||||||
| 271[M − H − Rha − Glc − H − CO − H]−; | ||||||||||
| 255[M − H − Rha − Glc − H − CO − OH]−; | ||||||||||
| 179[C8H3O5]−; | ||||||||||
| 151[C7H3O4]− | ||||||||||
| 37 | 23.74 | C21H20O12 | 464.08711 | 463.0874 | 0.6 | ― | ― | 463[M − H]−; | Isoquercitrin | c |
| 301[M − H − Glc]−; | ||||||||||
| 255[M − H − Glc − CO − H2O]−; | ||||||||||
| 151[C7H3O4]−; | ||||||||||
| 107[C6H3O2]− | ||||||||||
| 38 | 24.2 | C29H36O16 | 640.19197 | 639.19263 | 1 | ― | ― | 639[M − H]−; | Plantamajoside | e |
| 477[M − H − C9H6O3]−; | ||||||||||
| 315[M − H − C9H6O3 − Glc]−; | ||||||||||
| 153[M − H − C9H6O3 − 2Glc]−; | ||||||||||
| 135[M − H − C9H6O3 − 2Glc − H2O]− | ||||||||||
| 39 | 24.95 | C27H30O15 | 594.15009 | 593.1507 | 1 | ― | ― | 593[M − H]−; | Kaempferol-3-O-rutinoside | c |
| 327[M − H − C10H18O8]−; | ||||||||||
| 285[M − H − Rha − Glc]−; | ||||||||||
| 284[M − H − Rha − Glc − H]−; | ||||||||||
| 257[M − H − Rha − Glc − CO]−; | ||||||||||
| 255[M − H − Rha − Glc − H − CO − H]−; | ||||||||||
| 229[M − H − Rha − Glc − 2CO]−; | ||||||||||
| 227[M − H − Rha − Glc − H − 2CO − H]− | ||||||||||
| 40 | 25.34 | C28H36O8 | 500.24829 | ― | ― | 501.25002 | 3.4 | 501[M + H]+; | Tigloylgomisin H/Angeloygomi-sin H | d |
| 484[M + H − OH]+; | ||||||||||
| 483[M + H − H2O]+; | ||||||||||
| 384[M + H − OH − C4H7COOH]+; | ||||||||||
| 357[M + H − C4H7COOH − C2H4O]+ | ||||||||||
| 41 | 25.95 | C21H20O11 | 448.09219 | 447.09378 | 3.6 | ― | ― | 447[M − H]−; | Luteoloside | e |
| 285[M − H − Glc]−; | ||||||||||
| 284[M − H − Glc − H]−; | ||||||||||
| 257[M − H − Glc − CO]−; | ||||||||||
| 243[M − H − Glc − C2H2O]−; | ||||||||||
| 241[M − H − Glc − CO2]−; | ||||||||||
| 199[M − H − Glc − C2H2O − CO2]−; | ||||||||||
| 197[M − H − Glc − 2CO2]−; | ||||||||||
| 151[C7H3O4]− | ||||||||||
| 42 | 26.1 | C28H36O8 | 500.24829 | ― | ― | 501.25071 | 4.8 | 501[M + H]+; | Tigloylgomisin H/Angeloygomi-sin H | d |
| 484[M + H − OH]+; | ||||||||||
| 483[M + H − H2O]+; | ||||||||||
| 384[M + H − OH − C4H7COOH]+; | ||||||||||
| 357[M + H − C4H7COOH − C2H4O]+ | ||||||||||
| 43 | 26.57 | C29H36O15 | 624.19707 | 623.19604 | −1.6 | ― | ― | 623[M − H]−; | Acteoside | e |
| 461[M − H − C9H6O3]−; | ||||||||||
| 315[M − H − C9H6O3 − Rha]−; | ||||||||||
| 179[C9H7O4]−; | ||||||||||
| 153[M − H − C9H6O3 − Rha − Glc]−; | ||||||||||
| 135[M − H − C9H6O3 − Rha − Glc − H2O]− | ||||||||||
| 44 | 27.19 | C21H20O11 | 448.09219 | 447.09225 | 0.1 | 449.10532 | −2.5 | 447[M − H]−; | Astragalin | b,c |
| 285[M − H − Glc]−; | ||||||||||
| 151[C7H3O4]−; | ||||||||||
| 449[M + H]+; | ||||||||||
| 287[M − H − Glc]+; | ||||||||||
| 241[M − H − Glc − CH2O2]+; | ||||||||||
| 213[M + H − Glc − C2H2O3]+ | ||||||||||
| 45 | 27.19 | C21H20O11 | 448.09219 | 447.09225 | 0.1 | 449.10699 | −1.9 | 447[M − H]−; | Plantaginin | e |
| 285[M − H − Glc]−; | ||||||||||
| 267[M − H − Glc − H2O]−; | ||||||||||
| 255[M − H − Glc − CH2O]−; | ||||||||||
| 239[M − H − Glc − H2O − CO]−; | ||||||||||
| 167[C7H3O5]−; | ||||||||||
| 449[M + H]+; | ||||||||||
| 287[M+H − Glc]+; | ||||||||||
| 269[M + H − Glc − H2O]+; | ||||||||||
| 169[C7H5O5]+ | ||||||||||
| 46 | 27.48 | C29H36O15 | 624.19705 | 623.19794 | 1.4 | 625.21005 | −4.2 | 623[M − H]−; | Methylhesperidin | c |
| 477[M − H − Rha]−; | ||||||||||
| 315[M − H − Rha − Glc]−; | ||||||||||
| 300[M − H − Rha − Glc − CH3]−; | ||||||||||
| 297[M − H − Rha − Gldc − H2O]−; | ||||||||||
| 285[M − H − Rha − Glc − 2CH3]−; | ||||||||||
| 282[M − H − Rha − Glc − H2O − CH3]−; | ||||||||||
| 272[M − H − Rha − Glc − CH3 − CO]−; | ||||||||||
| 257[M − H − Rha − Glc − 2CH3 − CO]−; | ||||||||||
| 229[M − H − Rha − Glc − 2CH3 − 2CO]−; | ||||||||||
| 625[M + H]+; | ||||||||||
| 479[M + H − Rha]+; | ||||||||||
| 317[M + H − Rha − Glc]+; | ||||||||||
| 299[M + H − Rha − Glc − H2O]+; | ||||||||||
| 281[M + H − Rha − Glc − 2H2O]+; | ||||||||||
| 193[C10H9O4]+; | ||||||||||
| 165[C9H9O3]+ | ||||||||||
| 47 | 27.72 | C21H20O11 | 448.09219 | 447.09402 | 4.1 | ― | ― | 447[M − H]−; | Quercitrin | c |
| 301[M − H − Rha]−; | ||||||||||
| 300[M − H − Rha − H]−; | ||||||||||
| 273[M − H − Rha − CO]−; | ||||||||||
| 255[M − H − Rha − CO − H2O]− | ||||||||||
| 48 | 27.86 | C22H22O12 | 478.10275 | 477.10461 | 3.9 | ― | ― | 477[M − H]−; | Nepetin-7-O-glucoside | e |
| 315[M − H − Glc]−; | ||||||||||
| 287[M − H − Glc − CO]−; | ||||||||||
| 271[M − H − Glc − CO2]−; | ||||||||||
| 181[C8H5O5]− | ||||||||||
| 49 | 27.93 | C21H20O10 | 432.09727 | 431.09805 | 1.8 | 433.11093 | −4.6 | 431[M − H]−; | Kaempferol-7-O-α-l-rhamnoside | c |
| 413[M − H − H2O]−; | ||||||||||
| 285[M − H − Rha]−; | ||||||||||
| 267[M − H − Rha − H2O]−; | ||||||||||
| 169[C12H9O]−; | ||||||||||
| 155[C11H7O]−; | ||||||||||
| 433[M + H]+ | ||||||||||
| 50 | 27.97 | C21H20O10 | 432.09727 | 431.09866 | 3.2 | 431[M − H]−; | Apigenin-7-O-glucoside | e | ||
| 269[M − H − Glc]−; | ||||||||||
| 201[M − H − Glc − C3O2]−; | ||||||||||
| 151[C7H3O4]− | ||||||||||
| 51 | 27.99 | C15H16O9 | 340.07106 | 339.07058 | −1.4 | ― | ― | 339[M − H]−; | Esculin | c |
| 177[M − H − Glc]−; | ||||||||||
| 133[M − H − Glc − CO2]−; | ||||||||||
| 105[M − H − Glc − CO2 − CO]−; | ||||||||||
| 89[C7H5]− | ||||||||||
| 52 | 28.28 | C27H30O14 | 578.15518 | 577.15662 | 2.5 | 579.17073 | −0.2 | 577[M − H]−; | Rhoifolin | e |
| 457[M − H − C4H8O4]−; | ||||||||||
| 431[M − H − Rha]−; | ||||||||||
| 413[M − H − Rha − H2O]−; | ||||||||||
| 269[M − H − Rha − Glc]−; | ||||||||||
| 225[M − H − Rha − Glc − CO2]−; | ||||||||||
| 183[C12H7O2]−; | ||||||||||
| 579[M + H]+; | ||||||||||
| 433[M + H − Rha]+; | ||||||||||
| 415[M + H − Rha − H2O]+; | ||||||||||
| 397[M + H − Rha − 2H2O]+; | ||||||||||
| 271[M + H − Rha − Glc]+; | ||||||||||
| 253[M + H − Rha − Glc − H2O]+; | ||||||||||
| 243[M + H − Rha − Glc − CO]+; | ||||||||||
| 225[M + H − Rha − Glc − H2O − CO]+; | ||||||||||
| 211[M + H − Rha − Glc − H2O − C2H2O]+; | ||||||||||
| 153[C7H5O4]+; | ||||||||||
| 119[C8H7O]+; | ||||||||||
| 91[C7H7]+ | ||||||||||
| 53 | 28.28 | C28H32O16 | 624.16066 | 623.16248 | 2.9 | 625.17896 | 4.2 | 623[M − H]−; | Isorhamnetin-3-O-β-d-rutinoside | a |
| 477[M − H − Rha]−; | ||||||||||
| 315[M − H − Rha − Glc]−; | ||||||||||
| 300[M − H − Rha − Glc − CH3]−; | ||||||||||
| 272[M − H − Rha − Glc − CH3 − CO]−; | ||||||||||
| 271[M − H − Rha − Glc − CH3 − CO − H]−; | ||||||||||
| 243[M − H − Rha − Glc − CH3 − CO − H − CO]−; | ||||||||||
| 227[M − H − Rha − Glc − CH3 − CO − H − CO2]−; | ||||||||||
| 151[C7H3O4]−; | ||||||||||
| 625[M + H]+; | ||||||||||
| 479[M + H − Rha]+; | ||||||||||
| 317[M + H − Rha − Glc]+; | ||||||||||
| 302[M + H − Rha − Glc − CH3]+; | ||||||||||
| 285[M + H − Rha − Glc − CH3OH]+; | ||||||||||
| 274[M + H − Rha − Glc − CH3 − CO]+; | ||||||||||
| 257[M + H − Rha − Glc − CH3OH − CO]+; | ||||||||||
| 246[M + H − Rha − Glc − CH3 − 2CO]+; | ||||||||||
| 229[M + H − Rha − Glc − CH3OH − 2CO]+; | ||||||||||
| 153[C7H5O4]+ | ||||||||||
| 54 | 28.31 | C21H18O11 | 446.07654 | 445.07493 | −3.6 | ― | ― | 445[M − H]−; | Baicalin | e |
| 269[M − H − GluA]−; | ||||||||||
| 225[M − H − GluA − CO2]−; | ||||||||||
| 167[C7H3O5]− | ||||||||||
| 55 | 29.09 | C20H26O4 | 330.19039 | ― | ― | 331.18914 | −3.8 | 331[M + H]+; | Meso-dihydrog-uaiaretic acid | d |
| 313[M + H − H2O]+; | ||||||||||
| 301[M + H − 2CH3]+ | ||||||||||
| 56 | 29.58 | C15H10O5 | 270.0601 | 269.09566 | −1.6 | 271.05966 | −1.7 | 269[M − H]−; | Baicalein | e |
| 241[M − H − CO]−; | ||||||||||
| 225[M − H − CO2]−; | ||||||||||
| 197[M − H − CO − CO2]−; | ||||||||||
| 167[C7H3O5]−; | ||||||||||
| 271[M + H]+; | ||||||||||
| 253[M + H − H2O]+; | ||||||||||
| 169[C7H5O5]+ | ||||||||||
| 57 | 30.7 | C28H34O15 | 610.1814 | 609.18229 | 1.5 | 611.1994 | 3.9 | 609[M − H]−; | Neohesperidin | c |
| 505[M − H − C4H8O3]−; | ||||||||||
| 463[M − H − Rha]−; | ||||||||||
| 445[M − H − Rha − H2O]−; | ||||||||||
| 427[M − H − Rha − 2H2O]−; | ||||||||||
| 301[M − H − Rha − Glc]−; | ||||||||||
| 286[M − H − Rha − Glc − CH3]−; | ||||||||||
| 283[M − H − Rha − Glc − H2O]−; | ||||||||||
| 268[M − H − Rha − Glc − H2O − CH3]−; | ||||||||||
| 258[M − H − Rha − Glc − CH3 − CO]−; | ||||||||||
| 257[M − H − Rha − Glc − CO2]−; | ||||||||||
| 242[M − H − Rha − Glc − CH3 − CO2]−; | ||||||||||
| 239[M − H − Rha − Glc − H2O − CO2]−; | ||||||||||
| 199[C12H7O3]−; | ||||||||||
| 125[C6H5O3]−; | ||||||||||
| 611[M + H]+; | ||||||||||
| 303[M + H − Rha − Glc]+; | ||||||||||
| 285[M + H − Rha − Glc − H2O]+; | ||||||||||
| 179[C9H7O4]+; | ||||||||||
| 177[C10H9O3]+; | ||||||||||
| 151[C8H7O3]+ | ||||||||||
| 58 | 31.48 | C23H28O8 | 432.1857 | ― | ― | 433.18538 | −0.7 | 433[M + H]+; | Schizandradiol | d |
| 415[M + H − H2O]+; | ||||||||||
| 361[M + H − C4H8O]+; | ||||||||||
| 372[M + H − H2O − CH3CO]+; | ||||||||||
| 343[M + H − H2O − C4H7OH]+ | ||||||||||
| 59 | 31.54 39.44 | C15H10O5 | 270.04445 | 269.04353 | −3.4 | 271.06145 | 4.9 | 269[M − H]−; | Apigenin | e |
| 241[M − H − CO]−; | ||||||||||
| 227[M − H − C2H2O]−; | ||||||||||
| 225[M − H − CO2]−; | ||||||||||
| 201[M − H − C3O2]−; | ||||||||||
| 151[C7H3O4]−; | ||||||||||
| 271[M + H]+; | ||||||||||
| 253[M + H − H2O]+; | ||||||||||
| 225[M + H − H2O − CO]+ | ||||||||||
| 60 | 31.71 | C28H34O15 | 610.1814 | 609.18351 | 3.5 | 611.20001 | 4.8 | 609[M − H]−; | Hesperidin | c |
| 463[M − H − Rha]−; | ||||||||||
| 301[M − H − Rha − Glc]−; | ||||||||||
| 286[M − H − Rha − Glc − CH3]−; | ||||||||||
| 283[M − H − Rha − Glc − H2O]−; | ||||||||||
| 268[M − H − Rha − Glc − H2O − CH3]−; | ||||||||||
| 258[M − H − Rha − Glc − CH3 − CO]−; | ||||||||||
| 257[M − H − Rha − Glc − CO2]−; | ||||||||||
| 242[M − H − Rha − Glc − CH3 − CO2]−; | ||||||||||
| 239[M − H − Rha − Glc − H2O − CO2]−; | ||||||||||
| 199[C12H7O3]−; | ||||||||||
| 125[C6H5O3]−; | ||||||||||
| 611[M + H]+; | ||||||||||
| 303[M + H − Rha − Glc]+; | ||||||||||
| 285[M + H − Rha − Glc − H2O]+; | ||||||||||
| 179[C9H7O4]+; | ||||||||||
| 177[C10H9O3]+; | ||||||||||
| 151[C8H7O3]+ | ||||||||||
| 61 | 32.13 | C15H10O7 | 302.03428 | 301.03549 | 4 | 303.0488 | −1.1 | 301[M − H]−; | Quercetin | b,c |
| 273[M − H − CO]−; | ||||||||||
| 255[M − H − CO − H2O]−; | ||||||||||
| 151[C7H3O4]−; | ||||||||||
| 303[M + H]+; | ||||||||||
| 285[M + H − H2O]+; | ||||||||||
| 257[M + H − H2O − CO]+; | ||||||||||
| 247[M + H − 2CO]+; | ||||||||||
| 229[M + H − H2O − 2CO]+ | ||||||||||
| 62 | 33.02 | C30H26O13 | 594.12897 | 593.13049 | 2.6 | 595.14142 | −3.2 | 593[M − H]−; | Tiliroside | c |
| 447[M − H − C9H6O2]−; | ||||||||||
| 285[M − H − C9H6O2 − Glc]−; | ||||||||||
| 257[M − H − C9H6O2 − Glc − CO]−; | ||||||||||
| 229[M − H − C9H6O2 − Glc − 2CO]−; | ||||||||||
| 151[C7H3O4]−; | ||||||||||
| 617[M + Na]+; | ||||||||||
| 595[M + H]+; | ||||||||||
| 449[M + H − C9H6O2]+; | ||||||||||
| 287[M + H − C9H6O2 − Glc]+ | ||||||||||
| 63 | 34.12 | C15H12O6 | 288.05502 | 287.05549 | 1.7 | 289.07002 | −2.2 | 287[M − H]−; | Aromadendrin | c |
| 269[M − H − H2O]−; | ||||||||||
| 259[M − H − CO]−; | ||||||||||
| 243[M − H − CO2]−; | ||||||||||
| 225[M − H − H2O − CO2]−; | ||||||||||
| 215[M − H − CO − CO2]−; | ||||||||||
| 151[C7H3O4]−; | ||||||||||
| 107[C6H3O2]−; | ||||||||||
| 289[M + H]+; | ||||||||||
| 271[M + H − H2O]+; | ||||||||||
| 195[C9H7O5]+; | ||||||||||
| 145[C9H5O2]+ | ||||||||||
| 64 | 35.18 | C15H10O6 | 286.03936 | 285.03995 | 2.1 | 287.05359 | 4.9 | 285[M − H]−; | Kaempferol | b,c |
| 257[M − H − CO]−; | ||||||||||
| 229[M − H − 2CO]−; | ||||||||||
| 151[C7H3O4]−; | ||||||||||
| 309[M + Na]+; | ||||||||||
| 287[M + H]+; | ||||||||||
| 241[M + H − CH2O2]+; | ||||||||||
| 213[M + H − C2H2O3]+ | ||||||||||
| 65 | 35.31 | C15H10O6 | 286.03936 | 285.04019 | 2.9 | ― | ― | 571[2M − H]−; | Iuteolin | e |
| 285[M − H]−; | ||||||||||
| 243[M − H − C2H2O]−; | ||||||||||
| 241[M − H − CO2]−; | ||||||||||
| 199[M − H − C2H2O − CO2]−; | ||||||||||
| 197[M − H − 2CO2]−; | ||||||||||
| 171[M − H − C2H2O − CO2 − CO]−; | ||||||||||
| 151[C7H3O4]− | ||||||||||
| 66 | 35.58 | C16H12O7 | 316.04993 | 315.05117 | 3.9 | ― | ― | 315[M − H]−; | Nepetin | e |
| 287[M − H − CO]−; | ||||||||||
| 271[M − H − CO2]−; | ||||||||||
| 243[M − H − CO − CO2]−; | ||||||||||
| 181[C8H5O5]− | ||||||||||
| 67 | 35.61 | C15H10O6 | 286.03937 | 285.03989 | 1.8 | 287.05627 | 4.4 | 285[M − H]−; | Scutellarein | e |
| 257[M − H − CO]−; | ||||||||||
| 241[M − H − CO2]−; | ||||||||||
| 167[C7H3O5]−; | ||||||||||
| 287[M + H]+; | ||||||||||
| 269[M + H − H2O]+; | ||||||||||
| 241[M + H − H2O − CO]+; | ||||||||||
| 169[C7H5O5]+ | ||||||||||
| 68 | 35.61 | C16H12O7 | 316.04993 | 315.05027 | 1.1 | 317.06486 | −2.3 | 315[M − H]−; | Isorhamnetin | b |
| 300[M − H − CH3]−; | ||||||||||
| 272[M − H − CH3 − CO]−; | ||||||||||
| 271[M − H − CH3 − CO − H]−; | ||||||||||
| 243[M − H − CH3 − CO − H − CO]−; | ||||||||||
| 227[M − H − CH3 − CO − H − CO2]−; | ||||||||||
| 151[C7H3O4]−; | ||||||||||
| 107[C6H3O2]−; | ||||||||||
| 339[M + Na]+; | ||||||||||
| 317[M + H]+; | ||||||||||
| 302[M + H − CH3]+; | ||||||||||
| 285[M + H − CH3OH]+; | ||||||||||
| 274[M + H − CH3 − CO]+; | ||||||||||
| 257[M + H − CH3OH − CO]+; | ||||||||||
| 246[M + H − CH3 − 2CO]+; | ||||||||||
| 229[M + H − CH3OH − 2CO]+; | ||||||||||
| 153[C7H5O4]+ | ||||||||||
| 69 | 35.76 | C30H46O4 | 470.34689 | 469.33251 | 2.7 | 471.34509 | −3.8 | 469[M − H]−; | Nigranoic acid | d |
| 423[M − H − HCOOH]−; | ||||||||||
| 378[M − H − HCOOH − HCOO]−; | ||||||||||
| 471[M + H]+; | ||||||||||
| 456[M + H − CH3]+; | ||||||||||
| 453[M + H − H2O]+; | ||||||||||
| 390[M + H − C6H9]+ | ||||||||||
| 70 | 35.89 | C28H24O16 | 616.09807 | 615.10097 | 4.7 | ― | ― | 615[M − H]−; | 2″-O-Galloylhyperoside | c |
| 463[M − H − C7H4O4]−; | ||||||||||
| 445[M − H − C7H6O5]−; | ||||||||||
| 301[M − H − C7H4O4 − Glc]− | ||||||||||
| 71 | 36.79 | C23H30O7 | 418.20643 | ― | ― | 419.20477 | −4.0 | 419[M + H]+; | Gomisin H | d |
| 401[M + H − H2O]+; | ||||||||||
| 384[M + H − OH − H2O]+; | ||||||||||
| 383[M + H − 2H2O]+; | ||||||||||
| 369[M + H − OH − CH3 − H2O]+; | ||||||||||
| 353[M + H − OH − OCH3 − H2O]+ | ||||||||||
| 72 | 37.08 | C28H34O8 | 498.23264 | ― | ― | 499.23119 | −2.9 | 499[M + H]+; | Angeloygomisin O/Angeloylisogomisin O | d |
| 399[M + H − C4H7COOH]+; | ||||||||||
| 369[M + H − C4H7COOH − CH2O]+; | ||||||||||
| 357[M + H − C4H7COOH − C3H6]+; | ||||||||||
| 343[M + H − C4H7COOH − C4H8]+ | ||||||||||
| 73 | 38.12 | C28H34O8 | 498.23264 | ― | ― | 499.23026 | −4.8 | 499[M + H]+; | Angeloygomisin O/Angeloylisogomisin O | d |
| 399[M + H − C4H7COOH]+; | ||||||||||
| 369[M + H − C4H7COOH − CH2O]+; | ||||||||||
| 357[M + H − C4H7COOH − C3H6]+; | ||||||||||
| 343[M + H − C4H7COOH − C4H8]+ | ||||||||||
| 74 | 39.28 | C23H28O7 | 416.19078 | ― | ― | 417.18903 | −4.2 | 417[M + H]+; | Gomisin O/Epigomisin O | d |
| 402[M + H − CH3]+; | ||||||||||
| 399[M + H − H2O]+; | ||||||||||
| 385[M + H − CH3OH]+; | ||||||||||
| 373[M + H − C2H4O]+ | ||||||||||
| 75 | 39.38 | C24H32O7 | 432.22209 | ― | ― | 433.22103 | −2.4 | 433[M + H]+; | Schisandrin | d |
| 415[M + H − H2O]+; | ||||||||||
| 400[M + H − H2O − CH3]+; | ||||||||||
| 384[M + H − H2O − OCH3]+; | ||||||||||
| 373[M + H − H2O − C3H6]+; | ||||||||||
| 359[M + H − H2O − C4H8]+ | ||||||||||
| 76 | 39.44 | C30H46O4 | 470.34689 | ― | ― | 471.34551 | −2.9 | 471[M + H]+; | Kadsuric acid | d |
| 453[M + H − H2O]+; | ||||||||||
| 398[M + H − CH2CH2COOH]+; | ||||||||||
| 370[M + H − CH2CH2COOH − C2H4]+ | ||||||||||
| 77 | 39.6 | C23H28O7 | 416.19078 | ― | ― | 417.19103 | 0.6 | 417[M + H]+; | Gomisin O/Epigomisin O | d |
| 402[M + H − CH3]+; | ||||||||||
| 399[M + H − H2O]+; | ||||||||||
| 385[M + H − CH3OH]+; | ||||||||||
| 373[M + H − C2H4O]+ | ||||||||||
| 78 | 40.63 | C28H34O10 | 530.22247 | ― | ― | 531.22218 | −0.6 | 531[M + H]+; | Gomisin D | d |
| 485[M + H − CH2O2]+; | ||||||||||
| 401[M + H − C6H10O3]+; | ||||||||||
| 383[M + H − C6H10O3 − H2O]+ | ||||||||||
| 79 | 40.98 | C22H28O6 | 388.19586 | ― | ― | 389.19503 | −2.1 | 389[M + H]+; | Gomisin J | d |
| 374[M + H − CH3]+; | ||||||||||
| 357[M + H − CH3OH]+; | ||||||||||
| 342[M + H − CH3OH − CH3]+ | ||||||||||
| 80 | 41.02 | C30H48O5 | 488.3418 | 487.3439 | 4.3 | 489.35526 | −4.5 | 487[M − H]−; | Rosolic acid/2α,3α,19α-Trihydroxyolean-12-ene-28-oic acid/Arjunolic acid | c |
| 469[M − H − H2O]−; | ||||||||||
| 443[M − H − CO2]−; | ||||||||||
| 425[M − H − CO2 − H2O]−; | ||||||||||
| 407[M − H − CO2 − 2H2O]−; | ||||||||||
| 391[M − H − CO2 − 2H2O − CH4]−; | ||||||||||
| 489[M + H]+; | ||||||||||
| 453[M + H − 2H2O]+ | ||||||||||
| 81 | 41.22 | C30H48O5 | 488.3418 | 487.34378 | 4 | 489.35544 | −4.1 | 487[M − H]−; | Rosolic acid/2α,3α,19α-Trihydroxyolean-12-ene-28-oic acid/Arjunolic acid | c |
| 469[M − H − H2O]−; | ||||||||||
| 443[M − H − CO2]−; | ||||||||||
| 425[M − H − CO2 − H2O]−; | ||||||||||
| 407[M − H − CO2 − 2H2O]−; | ||||||||||
| 391[M − H − CO2 − 2H2O − CH4]−; | ||||||||||
| 489[M + H]+; | ||||||||||
| 453[M + H − 2H2O]+ | ||||||||||
| 82 | 41.75 | C15H24N2O2 | 264.10889 | ― | ― | 265.18997 | −4.0 | 265[M + H]+; | Sophoranol | b |
| 248[M + H − OH]+; | ||||||||||
| 247[M + H − H2O]+ | ||||||||||
| 83 | 42.18 | C30H46O3 | 454.35198 | ― | ― | 455.35025 | −3.8 | 455[M + H]+; | Ganwuweizic acid | d |
| 409[M + H − HCOOH]+; | ||||||||||
| 313[M + H − C8H14O2]+ | ||||||||||
| 84 | 42.32 | C30H48O5 | 488.3418 | 487.34302 | 2.5 | 489.35529 | −4.4 | 487[M − H]−; | Rosolic acid/2α,3α,19α-Trihydroxyolean-12-ene-28-oic acid/Arjunolic acid | c |
| 469[M − H − H2O]−; | ||||||||||
| 443[M − H − CO2]−; | ||||||||||
| 425[M − H − CO2 − H2O]−; | ||||||||||
| 407[M − H − CO2 − 2H2O]−; | ||||||||||
| 391[M − H − CO2 − 2H2O − CH4]−; | ||||||||||
| 489[M + H]+; | ||||||||||
| 453[M + H − 2H2O]+ | ||||||||||
| 85 | 42.89 | C12H16N2O | 204.13354 | ― | ― | 205.13409 | 2.6 | 205[M + H]+; | N-methylcytisine | b |
| 146[M + H − C3H9N]+; | ||||||||||
| 108[C6H6NO]+ | ||||||||||
| 86 | 43.27 | C23H28O6 | 400.19587 | ― | ― | 401.19434 | −3.8 | 401[M + H]+; | Schizandrin B | d |
| 386[M + H − CH3]+; | ||||||||||
| 370[M + H − OCH3]+; | ||||||||||
| 355[M + H − CH3 − OCH3]+; | ||||||||||
| 339[M + H − 2CH3 − CH3OH]+ | ||||||||||
| 87 | 43.3 | C30H34O8 | 522.23265 | ― | ― | 523.23095 | −3.2 | 523[M + H]+; | Benzoylgomisin H | d |
| 505[M + H − H2O]+; | ||||||||||
| 401[M + H − C6H5COOH]+; | ||||||||||
| 383[M + H − C6H5COOH − H2O]+ | ||||||||||
| 88 | 45.42 | C23H30O6 | 402.21151 | ― | ― | 403.21011 | −3.5 | 403[M + H]+; | Schisanhenol | d |
| 372[M + H − OCH3]+; | ||||||||||
| 371[M + H − CH3OH]+; | ||||||||||
| 356[M + H − CH3OH − CH3]+; | ||||||||||
| 340[M + H − OCH3 − CH3OH]+ | ||||||||||
| 89 | 45.44 | C23H28O7 | 416.19078 | ― | ― | 417.18957 | −2.9 | 417[M + H]+; | Schisandrol B | d |
| 399[M + H − H2O]+; | ||||||||||
| 343[M + H − C4H8 − H2O]+; | ||||||||||
| 307[M + H − 2CH3 − 2OCH3 − H2O]+ | ||||||||||
| 90 | 45.67 | C28H34O9 | 514.22755 | ― | ― | 515.22559 | −3.8 | 515[M + H]+; | Schisantherin B/Schisantherin C | d |
| 415[M + H − C4H7COOH]+; | ||||||||||
| 385[M + H − C4H7COOH − CH2O]+; | ||||||||||
| 367[M + H − C4H7COOH − CH2O − H2O]+; | ||||||||||
| 353[M + H − C4H7COOH − CH2O − CH3OH]+ | ||||||||||
| 91 | 46.06 | C30H32O9 | 536.21191 | ― | ― | 537.20981 | −3.9 | 537[M + H]+; | Schisantherin A | d |
| 415[M + H − C6H5COOH]+; | ||||||||||
| 397[M + H − C6H5COOH − H2O]+; | ||||||||||
| 373[M + H − C6H5COOH − C3H6]+ | ||||||||||
| 92 | 46.58 | C30H48O4 | 472.34689 | 471.34872 | 3.9 | 473.30911 | −3.7 | 471[M − H]−; | Corosolic acid/Maslinic acid | c |
| 453[M − H − H2O]−; | ||||||||||
| 427[M − H − CO2]−; | ||||||||||
| 391[M − H − CO2 − 2H2O]−; | ||||||||||
| 473[M + H]+; | ||||||||||
| 455[M + H − H2O]+; | ||||||||||
| 203[C15H23]+; | ||||||||||
| 105[C8H9]+ | ||||||||||
| 93 | 46.73 | C15H24N2O | 248.19614 | ― | ― | 249.19699 | 2.2 | 271[M + Na]+; | Matrine | b |
| 249[M+H]+; | ||||||||||
| 231[M + H − H2O]+; | ||||||||||
| 150[C10H16N]+; | ||||||||||
| 148[C10H14N]+ | ||||||||||
| 94 | 47.28 | C22H26O6 | 386.18022 | ― | ― | 387.17992 | −0.8 | 387[M + H]+; | Gomisin L1/Gomisin L2/Gomisin M1/Gomisin M2 | d |
| 372[M + H − CH3]+; | ||||||||||
| 369[M + H − H2O]+; | ||||||||||
| 357[M + H − CH2O]+; | ||||||||||
| 329[M + H − C2H2O2]+ | ||||||||||
| 95 | 47.52 | C30H48O4 | 472.34689 | 471.34863 | 3.7 | 473.36044 | −4.4 | 471[M − H]−; | Corosolic acid/Maslinic acid | c |
| 453[M − H − H2O]−; | ||||||||||
| 427[M − H − CO2]−; | ||||||||||
| 391[M − H − CO2 − 2H2O]−; | ||||||||||
| 473[M + H]+; | ||||||||||
| 455[M + H − H2O]+; | ||||||||||
| 203[C15H23]+; | ||||||||||
| 105[C8H9]+ | ||||||||||
| 96 | 47.53 | C22H26O6 | 386.18022 | ― | ― | 387.17847 | −4.5 | 387[M + H]+; | Gomisin L1/Gomisin L2/Gomisin M1/Gomisin M2 | d |
| 372[M + H − CH3]+; | ||||||||||
| 369[M + H − H2O]+; | ||||||||||
| 357[M + H − CH2O]+; | ||||||||||
| 329[M + H − C2H2O2]+ | ||||||||||
| 97 | 47.76 | C28H34O9 | 514.22755 | ― | ― | 515.22716 | −0.7 | 515[M + H]+; | Schisantherin B/Schisantherin C | d |
| 415[M + H − C4H7COOH]+; | ||||||||||
| 385[M + H − C4H7COOH − CH2O]+; | ||||||||||
| 367[M + H − C4H7COOH − CH2O − H2O]+; | ||||||||||
| 353[M + H − C4H7COOH − CH2O − CH3OH]+ | ||||||||||
| 98 | 48.01 | C30H48O3 | 456.36763 | 455.35424 | 4.9 | 457.36636 | −2.7 | 455[M − H]−; | Oleanolic acid/Ursolic acid | c,e |
| 407[M − H − HCHO − H2O]−; | ||||||||||
| 391[M − H − HCHO − H2O − CH4]−; | ||||||||||
| 479[M + Na]+; | ||||||||||
| 457[M + H]+; | ||||||||||
| 439[M + H − H2O]+; | ||||||||||
| 393[M + H − HCOOH − H2O]+ | ||||||||||
| 99 | 48.24 | C22H26O6 | 386.18022 | ― | ― | 387.17883 | −3.6 | 387[M + H]+; | Gomisin L1/Gomisin L2/Gomisin M1/Gomisin M2 | d |
| 372[M + H − CH3]+; | ||||||||||
| 369[M + H − H2O]+; | ||||||||||
| 357[M + H − CH2O]+; | ||||||||||
| 329[M + H − C2H2O2]+ | ||||||||||
| 100 | 48.4 | C30H48O3 | 456.36763 | 455.35394 | 4.3 | 457.36612 | −3.3 | 455[M − H]−; | Oleanolic acid/Ursolic acid | c,e |
| 407[M − H − HCHO − H2O]−; | ||||||||||
| 391[M − H − HCHO − H2O − CH4]−; | ||||||||||
| 479[M + Na]+; | ||||||||||
| 457[M + H]+; | ||||||||||
| 457[M + H − H2O]+; | ||||||||||
| 393[M + H − HCOOH − H2O]+ | ||||||||||
| 101 | 48.65 | C22H26O6 | 386.18022 | ― | ― | 387.17877 | −3.7 | 387[M + H]+; | Gomisin L1/Gomisin L2/Gomisin M1/Gomisin M2 | d |
| 372[M + H − CH3]+; | ||||||||||
| 369[M + H − H2O]+; | ||||||||||
| 357[M + H − CH2O]+; | ||||||||||
| 329[M + H − C2H2O2]+ | ||||||||||
| 102 | 49.79 | C24H32O6 | 416.22714 | ― | ― | 417.22649 | −1.6 | 417[M + H]+; | Deoxyschizan-drin | d |
| 402[M + H − CH3]+; | ||||||||||
| 386[M + H − OCH3]+; | ||||||||||
| 370[M + H − CH3 − CH3OH]+; | ||||||||||
| 347[M + H − CH3 − C4H7]+; | ||||||||||
| 316[M + H − C6H13O]+; | ||||||||||
| 286[M + H − C6H13O − 2CH3]+; | ||||||||||
| 273[M + H − C6H13O − CH3 − CO]+; | ||||||||||
| 227[M + H − C6H13O − CO − OCH3 − 2CH3]+ | ||||||||||
| 103 | 52.02 | C16H14O4 | 270.09649 | ― | ― | 271.09595 | −2.0 | 271[M + H]+; 203[M + H − C5H8]+; | Imperatorin | c |
| 175[M + H − C5H8 − CO]+; | ||||||||||
| 159[M + H − C5H8 − CO2]+ | ||||||||||
| 104 | 53.29 | C30H32O8 | 520.21701 | ― | ― | 521.21559 | −2.7 | 521[M + H]+; | Benzoylgomisin O/Benzoyisolgomisin O | d |
| 399[M + H − C6H5COOH]+; | ||||||||||
| 369[M + H − C6H5COOH − CH2O]+; | ||||||||||
| 357[M + H − C6H5COOH − C3H6]+; | ||||||||||
| 343 M + H − C6H5COOH − C4H8]+ | ||||||||||
| 105 | 53.43 | C22H24O6 | 384.16457 | ― | ― | 385.16406 | −1.3 | 385[M + H]+; | Schizandrin C | d |
| 370[M + H − CH3]+; | ||||||||||
| 355[M + H − CH2O]+ | ||||||||||
| 106 | 54.08 | C30H32O8 | 520.21701 | ― | ― | 521.21611 | −1.7 | 521[M + H]+; | Benzoylgomisin O/Benzoyisolgomisin O | d |
| 399[M + H − C6H5COOH]+; | ||||||||||
| 369[M + H − C6H5COOH − CH2O]+; | ||||||||||
| 357[M + H − C6H5COOH − C3H6]+; | ||||||||||
| 343 M + H − C6H5COOH − C4H8]+ | ||||||||||
2.2.1. Flavonoids


2.2.2. Phenylpropanoids


2.2.3. Organic Acids

2.2.4. Terpenoids
2.2.5. Alkaloids
2.2.6. Miscellaneous

3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation of WZYZW Samples
3.3. Instrumentation and Conditions
3.3.1. Liquid Chromatography Conditions
3.3.2. ESI-MS/MS Detection
3.4. Data Processing
| Time (min) | Flow (mL/min) | A (%) | B (%) |
|---|---|---|---|
| 0 | 0.300 | 99.0 | 1.0 |
| 1.0 | 0.300 | 99.0 | 1.0 |
| 12 | 0.300 | 91.0 | 9.0 |
| 17 | 0.300 | 88.0 | 12.0 |
| 17.5 | 0.300 | 86.5 | 13.5 |
| 23 | 0.300 | 86.5 | 13.5 |
| 33 | 0.300 | 70 | 30.0 |
| 35 | 0.300 | 60 | 40.0 |
| 50 | 0.300 | 35 | 65.0 |
| 52 | 0.300 | 35 | 65.0 |
| 55 | 0.300 | 1.0 | 99.0 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, W.Y.; Hou, J.J.; Long, H.L.; Yang, W.Z.; Liang, J.; Guo, D.A. TCM-base new drug discovery and development in China. Chin. J. Nat. Med. 2014, 12, 241–250. [Google Scholar] [PubMed]
- Li, W.F.; Jiang, J.G.; Chen, J. Chinese medicine and its modernization demands. Arch. Med. Res. 2008, 39, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Chan, K. Chinese medicinal materials and their interface with Western medical concepts. J. Ethnopharmacol. 2005, 96, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Zhu, C.Y.; Yuan, W.C.; Ma, J.; Yu, R.; Guo, J. Advances in research on pharmacological effects and clinical application of Wuzi Yanzong Pill. Liaoning J. Tradit. Chin. Med. 2015, 42, 1814–1816. [Google Scholar]
- An, Q.; Zou, L. A meta-analysis of the Chinese medicine Wuziyanwan for treatment of oligospermia and asthenospermia. Chin. J. Hum. Sex. 2015, 24, 84–89. [Google Scholar]
- Sun, Z.W.; Li, Y.Z.; Zhang, C.C. Research advances on pharmacological effects and clinical applications of Wu Zi Yan Zong Wan. Asia-Pac. Tradit. Med. 2010, 6, 179–181. [Google Scholar]
- Zhou, J.L.; Liu, W.; Tan, C.M.; Zhu, M.; Ma, S.C. Quality control study of Wuziyanzong Pills. Chin. Pharm. J. 2015, 50, 125–130. [Google Scholar]
- Zhou, J.L.; Liu, W.; Chen, B.L.; Zhu, M. Determination of scopoletin in Lycii Fructus and Wuzi Yanzong Pill by HPLC-Fluorescence detection. Chin. J. Mod. Appl. Pharm. 2015, 32, 482–486. [Google Scholar]
- Miao, L.; Chen, M.L.; Cao, J.; Sun, M.Q.; Liu, J.X. LC-MS determination of 9 constituents in active fraction of Wuziyanzong. Chin. J. Pharm. Anal. 2011, 31, 659–663. [Google Scholar]
- Liu, W.; Zhou, J.L.; Chen, B.L.; Zhu, M. Simultaneous determination of five ingredients in Wuzi Yanzong Pill by HPLC. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 74–78. [Google Scholar]
- Pan, H.Q.; Yang, W.Z.; Zhang, Y.B.; Yang, M.; Feng, R.H.; Wu, W.Y.; Guo, D.A. An integrated strategy for the systematic characterization and discovery of new indole alkaloids from Uncaria rhynchophylla by UHPLC/DA-D/LTQ-Orbitrap-MS. Anal. Bioanal. Chem. 2015, 407, 6057–6070. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Xu, H.Y.; Ma, Y.N.; Wang, X.G.; Shi, Y.; Huang, B.; Tang, S.H.; Zhang, Y.; Li, D.F.; Liang, R.X.; et al. Characterization and rapid identification of chemical constituents of NaoXinTong capsules by UHPLC-linear ion trap/Orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 104–118. [Google Scholar]
- Kouloura, E.; Skaltsounis, A.L.; Michel, S.; Halabalaki, M. Ion tree-based structure elucidation of acetophenone dimers (AtA) from Acronychia pedunculata and their identification in extracts by liquid chromatography electrospray ionization LTQ-Orbitrap mass spectrometry. J. Mass. Spectrom. 2015, 50, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Boix, N.; Piqué, E.; Gómez-Catalan, J.; Medina-Remon, A.; Sasot, G.; Mercader-Martí, M.; Llobet, J.M.; Lamuela-Raventos, R.M. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-Orbitrap-MS. Food. Chem. 2015, 181, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wang, F.; Zhang, H.; Lu, J.Q.; Qiao, Y.J. Rapid identification of polymethoxylated flavonoids in traditional Chinese medicines with a practical strategy of stepwise mass defect filtering coupled to diagnostic product ions analysis based on a hybrid LTQ-Orbitrap mass spectrometer. Phytochem. Anal. 2014, 25, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W. The performance and featured applications of LTQ Orbitrap velos, a hybrid high resolution mass spectrometer using electrostatic orbital mass analyzer coupled with dual pressure ion trap. Mod. Instrum. Med. Treat. 2010, 5, 5–19. [Google Scholar]
- Kumar, A.; Saini, G.; Nair, A.; Sharma, R. UPLC: A preeminent technique in pharmaceutical analysis. Acta Pol. Pharm. 2012, 69, 371–380. [Google Scholar] [PubMed]
- Jin, G.W.; Zhang, F.F.; Xue, X.Y.; Xiao, Y.S.; Xu, Q.; Liang, X.M. Application of Ultra-performance Liquid Chromatography in the separation and analysis of complicated system-Traditional Chinese Medicine. Mod. Tradit. Chin. Med. Mater. Med. World Sci. Technol. 2006, 8, 106–111. [Google Scholar]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, D.; Wang, J.; Zhang, B.; Xie, S.; Wang, Q.; Xu, K.; Lin, R. Analysis of Chemical Constituents in Wuzi-Yanzong-Wan by UPLC-ESI-LTQ-Orbitrap-MS. Molecules 2015, 20, 21373-21404. https://doi.org/10.3390/molecules201219765
Zou D, Wang J, Zhang B, Xie S, Wang Q, Xu K, Lin R. Analysis of Chemical Constituents in Wuzi-Yanzong-Wan by UPLC-ESI-LTQ-Orbitrap-MS. Molecules. 2015; 20(12):21373-21404. https://doi.org/10.3390/molecules201219765
Chicago/Turabian StyleZou, Dixin, Jinfeng Wang, Bo Zhang, Suhua Xie, Qing Wang, Kexin Xu, and Ruichao Lin. 2015. "Analysis of Chemical Constituents in Wuzi-Yanzong-Wan by UPLC-ESI-LTQ-Orbitrap-MS" Molecules 20, no. 12: 21373-21404. https://doi.org/10.3390/molecules201219765
APA StyleZou, D., Wang, J., Zhang, B., Xie, S., Wang, Q., Xu, K., & Lin, R. (2015). Analysis of Chemical Constituents in Wuzi-Yanzong-Wan by UPLC-ESI-LTQ-Orbitrap-MS. Molecules, 20(12), 21373-21404. https://doi.org/10.3390/molecules201219765





