Analysis of Phenolic and Cyclic Compounds in Plants Using Derivatization Techniques in Combination with GC-MS-Based Metabolite Profiling
Abstract
:1. Introduction
| Product | Supplier or Institution |
|---|---|
| Commercial MS Libraries | |
| NIST—NIST/EPA/NIH Mass Spectral Library | National Institute of Standards and Technology/Gaithersburg, MD, USA |
| Wiley—Wiley Registry of Mass Spectral Data | John Wiley & Sons, Inc./ Hoboken, NJ, USA |
| FiehnLib—Fiehn GC-MS Metabolomics RTL Library | Agilent Technologies, Inc./ Santa Clara, CA, USA |
| Public MS Libraries & Resources | |
| GMD—Golm Metabolome Database | Max Planck Institute of Molecular Plant Physiology/Golm, Potsdam, Germany |
| MassBank—High Quality Mass Spectral Database | National Bioscience Database Center/Tokyo, Japan |
| MetabolomeExpress—Public MSRI Libraries | Plant Energy Biology, ARC Centre of Excellence/Acton, Canberra, Australia |
| ReSpect—Riken MSn spectral database (LC/MS) | Metabolomics Research Division, RIKEN Plant Science Center,/Tsuruoka, Japan |
| Metlin—Metabolite and Tandem MS Database | Scripps Center for Metabolomics/La Jolla, CA, USA |
| HMDB—Human Metabolome Database | Genome Alberta & Genome Canada, University of Alberta/Edmonton, Canada |
| m/z CLOUD—Advanced Mass Spectral Database | HighChem Ltd. / Bratislava, Slovakia |
| NIST—NIST Chemistry WebBook | National Institute of Standards and Technology/Gaithersburg, MD, USA |
| Free GC/MS Analysis Software & Tools | |
| AMDIS—Automated Mass Spectral Deconvolution and Identification System | National Institute of Standards and Technology/Gaithersburg, MD, USA |
| Tagfinder—GC-MS analysis software (free upon request) | Max Planck Institute of Molecular Plant Physiology/Golm, Potsdam, Germany |
| MetaboliteDetector—Data deconvolution & analysis | TU Braunschweig, Germany |
| OpenChrom—Software for chromatography and MS | Dr. Philip Wenig/ Hamburg, Germany |
| Free GC/MS Alignment Tools | |
| Metalign—Processing of LC-MS and GC-MS data | Wageningen UR (University & Research centre)/Wageningen, The Netherlands |
| MZmine—Processing of LC-MS and GC-MS data | Turku Centre for Biotechnology/ Turku, Finland |
| MetaboAnalyst—Comprehensive tool suite for metabolomic data analysis | The Metabolomics Innovation Centre (TMIC)/University of Alberta, Canada |
| SpectConnect—GC-MS data alignment and analysis | Massachusetts Institute of Technology (MIT)/Boston, MA, USA |
2. GC-MS Profiling of Complex Chemical Matrices
2.1. Extraction Methods and Metabolite Coverage
2.2. Derivatization of Metabolites

2.3. GC-MS Metabolite Profiling—Applications, Performance and Reliability
2.4. Secondary Metabolites in GC-MS-Based Metabolomic Approaches
3. Detection of Plant Phenolics and Other Cyclic Structures
3.1. Simple Phenolics, Aromatic Acids and Related Structures

3.2. Polyphenols

3.3. Terpenoids and Sterols

3.4. N-Containing Cyclic Structures

3.5. O-Containing Cyclic Structures
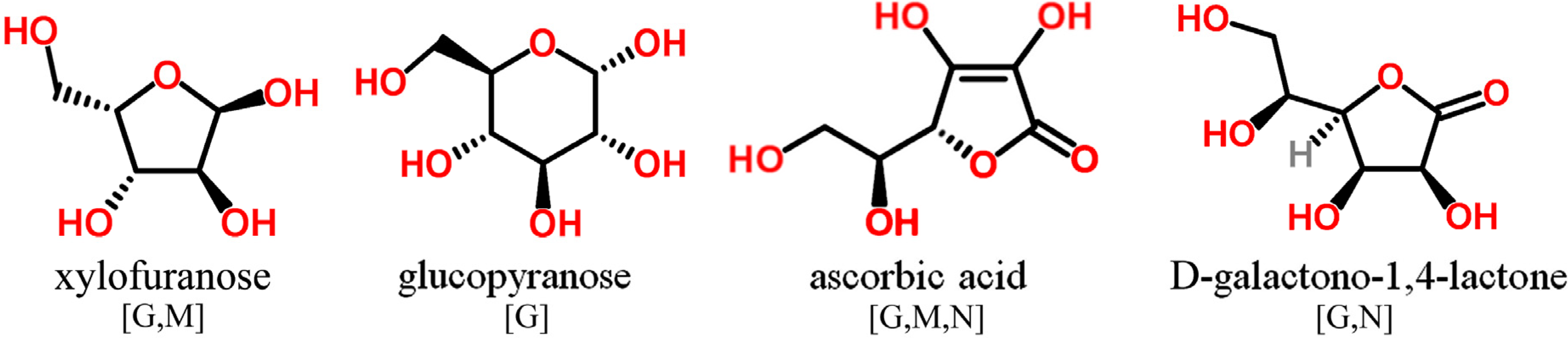
3.6. S-Containing Cyclic Structures

4. Case Studies—GC-MS Profiling of Plant Samples
4.1. Fresh Plant Samples: Flavonoids and Derivatives


4.2. Plant-Based Aquafeeds: Phenolic Acids


4.3. Cereals: Alkyresorcinols

4.4. Olive Oil: Simple Phenolic Structures and Secoiridoids

| RT | RI | Compound | Masses | DB | Mean | Low | High |
|---|---|---|---|---|---|---|---|
| 15.05 | 1238 | phenylethyl alcohol | M+ 194(1), 73(100), 103(81), 179(68), 105(24) | [N] | 0.19 | 0.04 | 0.82 |
| 15.40 | 1249 | 3,5-dimethylphenol | M+ 194(39), 179(100), 194(67), 105(16) | [N] | 0.28 | 0.03 | 1.45 |
| 15.64 | 1257 | benzoic acid | M+ 194(4), 179(100), 105(79), 135(62), 77(56) | [G,H,M,N] | 0.44 | 0.02 | 1.32 |
| 17.13 | 1306 | phenylacetic acid | M+ 208, 73(100), 164(21), 193(9), 137(3) | [G,H,M,N] | 0.26 | 0.04 | 0.87 |
| 17.80 | 1329 | catechol | M+ 254(100), 239(30), 151(20), 136(15), 166(13) | [G,H,M,N] | 5.15 | 0.45 | 28.52 |
| 20.34 | 1419 | hydrocinnamic acid | M+ 222(24), 104(100), 207(46), 91(26), 132(4) | [G,H,M,N] | 0.60 | 0.01 | 3.25 |
| 21.62 | 1466 | hydroxybenzoic acid | M+ 282(48), 73(100), 267(18), 179(14), 193(10) | [G,H,M] | 0.47 | 0.03 | 2.36 |
| 21.82 | 1473 | (E)-isoeugenol | M+ 236(42), 206(100), 73(21), 221(19), 179(11), 103(6) | [N] | 0.81 | 0.02 | 4.65 |
| 22.86 | 1513 | salicylic acid | M+ 282, 73(100), 267(92), 135(10), 193(5) | [G,H,N] | 0.17 | 0.01 | 0.49 |
| 23.83 | 1551 | syringaldehyde | M+ 254(45), 224(100), 73(79), 209(45), 239(33) | [N] | 0.04 | 0.03 | 0.07 |
| 24.26 | 1569 | p-tyrosol | M+ 282(19), 179(100), 267(13), 193(12) | [H] | 7.02 | 1.47 | 18.32 |
| 25.23 | 1608 | ligstroside deriv. | M+ 192(100), 177(67), 179(24), 193(19) | – | 4.76 | 0.04 | 18.91 |
| 25.62 | 1624 | methyl homovanillic acid | M+ 268(55), 73(100), 238(75), 209(46), 253(30) | – | 0.05 | 0.02 | 0.14 |
| 25.95 | 1639 | vanillin | M+ 253(71), 223(100), 73(29), 238(22), 165(12) | [N] | 1.28 | 0.03 | 6.12 |
| 27.45 | 1703 | homovanillyl alcohol | M+ 312(33), 73(100), 209(94), 103(22), 179(16) | [N] | 0.61 | 0.01 | 3.05 |
| 28.49 | 1749 | phloretic acid | M+ 308(52), 73(100), 219(71), 293(65), 249(53) | [N] | 0.11 | 0.04 | 0.46 |
| 28.63 | 1755 | vanillic acid | M+ 312(24), 267(49), 297(44), 282(34), 253(28) | [G,H,N] | 0.43 | 0.02 | 2.26 |
| 28.76 | 1761 | hydroxytyrosol | M+ 370(44), 267(100), 193(19), 179(11) | – | 1.47 | 0.27 | 5.35 |
| 29.77 | 1807 | oleuropein deriv. | M+ 340(13), 73(100), 280(96), 193(40), 179(14) | – | 7.19 | 0.17 | 35.98 |
| 30.75 | 1853 | p-coumaric acid | M+ 308(35), 73(100), 293(53), 219(42), 249(23) | [G,H,M,N] | 0.04 | 0.02 | 0.06 |
| 31.44 | 1887 | syringic acid | M+ 342(24), 327(100), 73(67), 312(64), 297(58) | [N] | 0.04 | 0.01 | 0.07 |
| 32.11 | 1919 | (Z)-ferulic acid | M+ 338(49), 73(100), 308(43), 323(37), 249(33) | [G,N] | 0.08 | 0.02 | 0.26 |
| 32.14 | 1921 | (E)-coniferaldehyde | M+ 279(27), 73(100), 248(56), 218(49), 232(14) | [G] | 0.06 | 0.04 | 0.15 |
| 43.86 | 2596 | ligstroside deriv.* | 192(100), 177(26), 179(8) | – | 0.13 | 0.05 | 0.60 |
| 44.12 | 2613 | ligstroside deriv.* | 192(100), 177(22) | – | 0.17 | 0.04 | 0.49 |
| 44.41 | 2633 | ligstroside aglycone (aldehydic form I) | M+ 434(5), 192(100), 177(19), 179(7), 403(3) | – | 4.42 | 0.07 | 25.91 |
| 44.57 | 2644 | ligstroside aglycone (aldehydic form II) | M+ 434(5), 192(100), 177(23), 179(7), 403(3) | – | 0.59 | 0.07 | 3.15 |
| 45.48 | 2706 | ligstroside aglycone deriv.* | 192(100), 177(15), 179(5) | – | 0.34 | 0.03 | 1.51 |
| 46.08 | 2749 | ligstroside aglycone deriv.* | 192(100), 177(22), 179(5) | – | 0.49 | 0.04 | 1.96 |
| 46.39 | 2770 | oleuropein deriv.* | 280(100), 193(35), 179(14) | – | 0.09 | 0.02 | 0.36 |
| 46.66 | 2790 | oleuropein deriv.* | 280(100), 192(74), 177(16) | – | 0.08 | 0.02 | 0.27 |
| 46.92 | 2809 | oleuropein aglycone (aldehydic form) | M+ 522(3), 280(100), 193(18), 179(4), 267(3) | – | 3.48 | 0.07 | 20.45 |
| 47.00 | 2814 | ligstroside deriv. | M+ 492(3), 192(100), 177(29), 280(13), 209(4), 461(2) | – | 0.81 | 0.03 | 4.66 |
| 47.08 | 2820 | ligstroside deriv. | M+ 492(2), 192(100), 177(30), 461(21), 209(14), 280(9) | – | 0.52 | 0.03 | 2.91 |
| 47.32 | 2837 | ligstroside deriv. | M+ 492(1), 192(100), 177(16), 179(5), 209(2), 280(1) | – | 2.57 | 0.07 | 14.28 |
| 47.67 | 2863 | ligstroside deriv.* | 192(100), 177(18), 355(10), 179(8) | – | 0.23 | 0.03 | 1.15 |
| 47.77 | 2870 | oleuropein deriv.* | 280(100), 193(26), 179(4) | – | 0.25 | 0.01 | 1.22 |
| 47.91 | 2881 | ligstroside deriv.* | 192(100), 177(38), 179(19) | – | 0.08 | 0.02 | 0.26 |
| 47.98 | 2886 | ligstroside deriv.* | 192(100), 177(16), 179(6) | – | 0.46 | 0.03 | 2.27 |
| 48.07 | 2893 | ligstroside deriv.* | 192(100), 177(18), 179(9) | – | 0.20 | 0.02 | 1.01 |
| 48.40 | 2918 | oleuropein deriv.* | 280(100), 193(23), 179(6), 267(4), 519(4) | – | 0.73 | 0.04 | 3.82 |
| 48.63 | 2935 | oleuropein deriv.* | 280(100), 193(44), 192(41) | – | 0.05 | 0.01 | 0.08 |
| 49.07 | 2968 | oleuropein deriv.* | 280(100), 193(28), 179(8), 355(7) | – | 0.14 | 0.02 | 0.66 |
| 49.13 | 2973 | oleuropein deriv.* | 280(100), 193(28), 179(14) | – | 0.10 | 0.02 | 0.37 |
| 49.22 | 2979 | oleuropein deriv. | M+ 580(2), 280(100), 193(21), 179(5), 267(4) | – | 0.33 | 0.03 | 1.80 |
| 49.27 | 2984 | oleuropein deriv. | M+ 549(5), 280(100), 193(27), 179(8), 267(4) | – | 0.31 | 0.03 | 1.68 |
| 49.35 | 2989 | oleuropein deriv. | M+ 551(2), 280(100), 193(21), 179(4), 519(1) | – | 0.88 | 0.04 | 4.94 |
| 49.46 | 2998 | oleuropein deriv. | M+ 551(2), 280(100), 193(19), 179(4), 519(1) | – | 6.32 | 0.08 | 36.78 |
| 49.79 | 3023 | oleuropein deriv. | M+ 551(2), 280(100), 193(18), 179(5), 267(4) | – | 0.47 | 0.02 | 2.62 |
| 49.98 | 3038 | oleuropein deriv.* | 280(100), 355(30), 193(20), 368(4) | – | 0.30 | 0.02 | 1.60 |
| 50.04 | 3043 | oleuropein deriv.* | 280(100), 193(18), 179(5), 355(2) | – | 0.64 | 0.04 | 3.43 |
| 50.15 | 3051 | oleuropein deriv.* | 280(100), 193(17), 179(7), 267(5) | – | 0.23 | 0.01 | 1.16 |
| 51.78 | 3182 | luteolin | M+ 559(100), 73(54), 487(8), 272(7) | [G,H] | 0.05 | 0.01 | 0.09 |
| 53.26 | 3306 | pinoresinol | M+ 502(59), 223(100), 73(75), 209(56), 235(43) | – | 0.08 | 0.02 | 0.16 |
| 53.34 | 3313 | β-sitosterol | M+ 486(25), 129(100), 357(97), 396(92), 73(63), 381(40) | [G,H,N] | 0.12 | 0.03 | 0.24 |
| 53.76 | 3348 | acetoxipinoresinol | M+ 560(18), 276(100), 245(53), 73(37), 209(34), 261(20) | – | 1.06 | 0.04 | 5.40 |
| 55.59 | 3509 | uvaol | M+ 496(89), 216(100), 73(58), 203(51), 188(25), 161(24) | – | 0.32 | 0.02 | 0.96 |
| 56.65 | 3607 | oleanolic acid | M+ 585(6), 203(100), 73(53), 320(35), 189(33), 482(24) | [G,H] | 1.36 | 0.05 | 3.77 |
| Total Phenolics | 59.96 | 4.22 | 286.99 | ||||
| oleuropein structures | 21.61 | 0.72 | 117.21 | ||||
| ligstroside structures | 15.78 | 0.52 | 79.08 | ||||
| tyrosols | 8.49 | 1.74 | 23.67 | ||||
| phenolic acids | 2.69 | 0.36 | 11.56 | ||||
| alcohols | 7.04 | 0.58 | 38.49 | ||||
| aldehydes | 1.37 | 0.10 | 6.34 | ||||
| lignans | 1.13 | 0.07 | 5.56 | ||||
| flavonoids | 0.05 | 0.03 | 0.09 |
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar]
- Fiehn, O. Validated high quality automated metabolome analysis of Arabidopsis thaliana leaf disks. In Concepts in Plant Metabolomics; Nikolau, B.J., Wurtele, E.S., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–18. [Google Scholar]
- Hall, R.; Beale, M.; Fiehn, O.; Hardy, N.; Sumner, L.; Bino, R. Plant metabolomics: The missing link in functional genomics strategies. Plant Cell 2002, 14, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Halket, J.M.; Waterman, D.; Przyborowska, A.M.; Patel, R.K.; Fraser, P.D.; Bramley, P.M. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J. Exp. Bot. 2005, 56, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.Y.; Choi, Y.H.; Verpoorte, R.; Wilson, E.G. Extraction for metabolomics: Access to the metabolome. Phytochem. Anal. PCA 2014, 25, 291–306. [Google Scholar] [CrossRef]
- Frenzel, T.; Miller, A.; Engel, K.H. Metabolite profiling—A Fractionation method for analysis of major and minor compounds in rice grains. Cereal Chem. 2002, 79, 215–221. [Google Scholar] [CrossRef]
- Rohloff, J. Cultivation of herbs and medicinal plants in Norway—Essential oil production and quality control. PhD thesis, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, April 2003. [Google Scholar]
- Weldegergis, B.T.; Crouch, A.M. Analysis of volatiles in Pinotage wines by stir bar sorptive extraction and chemometric profiling. J. Agric. Food Chem. 2008, 56, 10225–10236. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, J.; Li, D. Microextraction techniques for the determination of volatile and semivolatile organic compounds from plants: A review. Anal. Chim. Acta 2013, 799, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Cevallos, J.M.; Garcia-Torres, R.; Etxeberria, E.; Reyes-De-Corcuera, J.I. GC-MS Analysis of Headspace and Liquid Extracts for Metabolomic Differentiation of Citrus Huanglongbing and Zinc Deficiency in Leaves of “Valencia” Sweet Orange from Commercial Groves. Phytochem. Anal. 2011, 22, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Ito, R.; Nakazawa, H.; Takatsu, A. Applications of stir-bar sorptive extraction to food analysis. TrAC Trends Anal. Chem. 2013, 45, 280–293. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Nouri, N.; Khorram, P. Derivatization and microextraction methods for determination of organic compounds by gas chromatography. Trac Trends Anal. Chem. 2014, 55, 14–23. [Google Scholar] [CrossRef]
- Bojko, B.; Reyes-Garces, N.; Bessonneau, V.; Gorynski, K.; Mousavi, F.; Silva, E.A.S.; Pawliszyn, J. Solid-phase microextraction in metabolomics. Trac-Trend Anal. Chem. 2014, 61, 168–180. [Google Scholar] [CrossRef]
- Tikunov, Y.; Lommen, A.; de Vos, C.H.; Verhoeven, H.A.; Bino, R.J.; Hall, R.D.; Bovy, A.G. A novel approach for nontargeted data analysis for metabolomics. Large-scale profiling of tomato fruit volatiles. Plant Physiol. 2005, 139, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.; Besada, C.; Badenes, M.L.; Monforte, A.J.; Granell, A. A non-targeted approach unravels the volatile network in peach fruit. PLoS One 2012, 7, e38992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkemeyer, C.; Kolasa, A.; Kopka, J. Comprehensive chemical derivatization for gas chromatography-mass spectrometry-based multi-targeted profiling of the major phytohormones. J. Chromatogr. A 2003, 993, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Little, J.L. Artifacts in trimethylsilyl derivatization reactions and ways to avoid them. J. Chromatogr. A 1999, 844, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Villas-Boas, S.G.; Smart, K.F.; Sivakumaran, S.; Lane, G.A. Alkylation or silylation for analysis of amino and non-amino organic acids by GC-MS? Metabolites 2011, 1, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Wohlgemuth, G.; Lee do, Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Kopka, J.; Dormann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Technical advance: Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Gang, D.R.; Charlton, A.J.; Fiehn, O.; Kuiper, H.A.; Reynolds, T.L.; Tjeerdema, R.S.; Jeffery, E.H.; German, J.B.; Ridley, W.P.; et al. Applications of metabolomics in agriculture. J. Agric. Food Chem. 2006, 54, 8984–8994. [Google Scholar] [CrossRef] [PubMed]
- Schauer, N.; Zamir, D.; Fernie, A.R. Metabolic profiling of leaves and fruit of wild species tomato: A survey of the Solanum lycopersicum complex. J. Exp. Bot. 2005, 56, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Schauer, N.; Semel, Y.; Roessner, U.; Gur, A.; Balbo, I.; Carrari, F.; Pleban, T.; Perez-Melis, A.; Bruedigam, C.; Kopka, J.; et al. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat. Biotechnol. 2006, 24, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk-Wochniak, E.; Fernie, A.R. Metabolic profiling reveals altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. J. Exp. Bot. 2005, 56, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Carrari, F.; Baxter, C.; Usadel, B.; Urbanczyk-Wochniak, E.; Zanor, M.I.; Nunes-Nesi, A.; Nikiforova, V.; Centero, D.; Ratzka, A.; Pauly, M.; et al. Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiol. 2006, 142, 1380–1396. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Sefkow, M.; Kopka, J. Construction and application of a mass spectral and retention time index database generated from plant GC/EI-TOF-MS metabolite profiles. Phytochemistry 2003, 62, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Bernards, M.A. Metabolite profiling of potato (Solanum tuberosum L.) tubers during wound-induced suberization. Metabolomics 2007, 3, 147–159. [Google Scholar] [CrossRef]
- Röhlig, R.M.; Eder, J.; Engel, K.H. Metabolite profiling of maize grain: Differentiation due to genetics and environment. Metabolomics 2009, 5, 459–477. [Google Scholar] [CrossRef]
- Benkeblia, N.; Shinano, T.; Osaki, M. Metabolite profiling and assessment of metabolome compartmentation of soybean leaves using non-aqueous fractionation and GC-MS analysis. Metabolomics 2007, 3, 297–305. [Google Scholar] [CrossRef]
- Dauwe, R.; Morreel, K.; Goeminne, G.; Gielen, B.; Rohde, A.; van Beeumen, J.; Ralph, J.; Boudet, A.M.; Kopka, J.; Rochange, S.F.; et al. Molecular phenotyping of lignin-modified tobacco reveals associated changes in cell-wall metabolism, primary metabolism, stress metabolism and photorespiration. Plant J. 2007, 52, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Lytovchenko, A.; Beleggia, R.; Schauer, N.; Isaacson, T.; Leuendorf, J.E.; Hellmann, H.; Rose, J.K.; Fernie, A.R. Application of GC-MS for the detection of lipophilic compounds in diverse plant tissues. Plant Methods 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Stamova, B.S.; Roessner, U.; Suren, S.; Laudencia-Chingcuanco, D.; Bacic, A.; Beckles, D.M. Metabolic profiling of transgenic wheat over-expressing the high-molecular-weight Dx5 glutenin subunit. Metabolomics 2009, 5, 239–252. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, C.F.; Xu, H.L.; Yuan, K.L.; Lu, X.; Zhu, Z.; Wu, Y.N.; Xu, G.W. Metabolic profiling of transgenic rice with cryIAc and sck genes: An evaluation of unintended effects at metabolic level by using GC-FID and GC-MS. J. Chromatogr. B 2009, 877, 725–732. [Google Scholar] [CrossRef]
- Sissener, N.H.; Hemre, G.-I.; Lall, S.P.; Sagstad, A.; Petersen, K.; Williams, J.; Rohloff, J.; Sanden, M. Are apparent negative effects of feeding genetically modified MON810 maize to Atlantic salmon, Salmo salar caused by confounding factors? Br. J. Nutr. 2011, 106, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Koek, M.M.; van der Kloet, F.M.; Kleemann, R.; Kooistra, T.; Verheij, E.R.; Hankemeier, T. Semi-automated non-target processing in GC × GC-MS metabolomics analysis: Applicability for biomedical studies. Metabolomics 2011, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Roessner-Tunali, U.; Hegemann, B.; Lytovchenko, A.; Carrari, F.; Bruedigam, C.; Granot, D.; Fernie, A.R. Metabolic profiling of transgenic tomato plants overexpressing hexokinase reveals that the influence of hexose phosphorylation diminishes during fruit development. Plant Physiol. 2003, 133, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Dethloff, F.; Erban, A.; Orf, I.; Alpers, J.; Fehrle, I.; Beine-Golovchuk, O.; Schmidt, S.; Schwachtje, J.; Kopka, J. Profiling Methods to Identify Cold-Regulated Primary Metabolites Using Gas Chromatography Coupled to Mass Spectrometry. In Plant Cold Acclimation: Methods and Protocols; Hincha, D.K., Zuther, E., Eds.; Humana Press: New York, NY, USA, 2014; Volume 1166, pp. 171–197. [Google Scholar]
- Isidorov, V.A.; Szczepaniak, L. Gas chromatographic retention indices of biologically and environmentally important organic compounds on capillary columns with low-polar stationary phases. J. Chromatogr. A 2009, 1216, 8998–9007. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef] [PubMed]
- Saitta, M.; Salvo, F.; di Bella, G.; Dugo, G.; la Torre, G.L. Minor compounds in the phenolic fraction of virgin olive oils. Food Chem. 2009, 112, 525–532. [Google Scholar] [CrossRef]
- Zhang, K.; Zuo, Y. GC-MS determination of flavonoids and phenolic and benzoic acids in human plasma after consumption of cranberry juice. J. Agric. Food Chem. 2004, 52, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Wang, C.; Zhan, J. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC-MS. J. Agric. Food Chem. 2002, 50, 3789–3794. [Google Scholar] [CrossRef] [PubMed]
- Füzfai, Z.; Molnár-Perl, I. Gas chromatographic-mass spectrometric fragmentation study of flavonoids as their trimethylsilyl derivatives: Analysis of flavonoids, sugars, carboxylic and amino acids in model systems and in citrus fruits. J. Chromatogr. A 2007, 1149, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Williams, S.J.; Woodman, O.L.; Marriott, P.J. Comprehensive two-dimensional gas chromatography, retention indices and time-of-flight mass spectra of flavonoids and chalcones. J. Chromatogr. A 2010, 1217, 8317–8326. [Google Scholar] [CrossRef] [PubMed]
- Zarnowski, R.; Suzuki, Y.; Yamaguchi, I.; Pietr, S.J. Alkylresorcinols in barley (Hordeum vulgare L. distichon) grains. Z. Naturforsch. C 2002, 57, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jung, B.H.; Kim, S.Y.; Chung, B.C. Determination of phytoestrogens in traditional medicinal herbs using gas chromatography-mass spectrometry. J. Nutr. Biochem. 2004, 15, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.J.; Gil, M.J.; Gutierrez-Rosales, F. Solid-phase extraction gas chromatography-ion trap-mass spectrometry qualitative method for evaluation of phenolic compounds in virgin olive oil and structural confirmation of oleuropein and ligstroside aglycons and their oxidation products. J. Chromatogr. A 2005, 1093, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.P.; Graikou, K.; Chinou, I.; Bankova, V.S. GC-MS profiling of diterpene compounds in Mediterranean propolis from Greece. J. Agric. Food Chem. 2010, 58, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Razborsek, M.I.; Voncina, D.B.; Dolecek, V.; Voncina, E. Determination of major phenolic acids, phenolic diterpenes and triterpenes in rosemary (Rosmarinus officinalis L.) by gas chromatography and mass spectrometry. Acta Chim. Slov. 2007, 54, 60–67. [Google Scholar]
- Esche, R.; Barnsteiner, A.; Scholz, B.; Engel, K.H. Simultaneous analysis of free phytosterols/phytostanols and intact phytosteryl/phytostanyl fatty acid and phenolic acid esters in cereals. J. Agric. Food Chem. 2012, 60, 5330–5339. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.C.; Pegg, R.B.; Phillips, R.D.; Eitenmiller, R.R. Commercial peanut (Arachis hypogaea L.) cultivars in the United States: Phytosterol composition. J. Agric. Food Chem. 2010, 58, 9137–9146. [Google Scholar] [CrossRef] [PubMed]
- Meagher, L.P.; Beecher, G.R.; Flanagan, V.P.; Li, B.W. Isolation and characterization of the lignans, isolariciresinol and pinoresinol, in flaxseed meal. J. Agric. Food Chem. 1999, 47, 3173–3180. [Google Scholar] [CrossRef] [PubMed]
- Lamikanra, O.; Grimm, C.C.; Rodin, J.B.; Inyang, I.D. Hydroxylated stilbenes in selected American wines. J. Agric. Food Chem. 1996, 44, 1111–1115. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Torras-Claveria, L.; Berkov, S.; Jauregui, O.; Caujape, J.; Viladomat, F.; Codina, C.; Bastida, J. Metabolic profiling of bioactive Pancratium canariense extracts by GC-MS. Phytochem. Anal. PCA 2010, 21, 80–88. [Google Scholar] [CrossRef]
- Kopka, J. Current challenges and developments in GC-MS based metabolite profiling technology. J. Biotechnol. 2006, 124, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Kotowska, U.; Vinogorova, V.T. GC identification of organic compounds based on partition coefficients of their TMS derivatives in a hexane-acetonitrile sytem and retention indices. Anal. Sci. 2005, 21, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Vinogorova, V.T. GC-MS analysis of compounds extracted from buds of Populus balsamifera and Populus nigra. Z. Fur Naturforschung C J. Biosci. 2003, 58, 355–360. [Google Scholar]
- Schauer, N.; Steinhauser, D.; Strelkov, S.; Schomburg, D.; Allison, G.; Moritz, T.; Lundgren, K.; Roessner-Tunali, U.; Forbes, M.G.; Willmitzer, L.; et al. GC-MS libraries for the rapid identification of metabolites in complex biological samples. FEBS Lett. 2005, 579, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. JMS 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Carroll, A.J.; Badger, M.R.; Harvey Millar, A. The MetabolomeExpress Project: Enabling web-based processing, analysis and transparent dissemination of GC/MS metabolomics datasets. BMC Bioinform. 2010, 11. [Google Scholar] [CrossRef]
- Kamthan, A.; Kamthan, M.; Chakraborty, N.; Chakraborty, S.; Datta, A. A simple protocol for extraction, derivatization, and analysis of tomato leaf and fruit lipophilic metabolites using GC-MS. Protocol. Exch. 2012. [Google Scholar] [CrossRef]
- Shuman, J.L.; Cortes, D.F.; Armenta, J.M.; Pokrzywa, R.M.; Mendes, P.; Shulaev, V. Plant metabolomics by GC-MS and differential analysis. In Plant Reverse Genetics: Methods and Protocols; Pereira, A., Ed.; Springer: New York, NY, USA, 2010; Volume 678, pp. 229–246. [Google Scholar]
- Cuthbertson, D.J.; Johnson, S.R.; Piljac-Zegarac, J.; Kappel, J.; Schafer, S.; Wust, M.; Ketchum, R.E.; Croteau, R.B.; Marques, J.V.; Davin, L.B.; et al. Accurate mass-time tag library for LC/MS-based metabolite profiling of medicinal plants. Phytochemistry 2013, 91, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Yamada, Y.; Sawada, Y.; Matsuda, F.; Akiyama, K.; Shinozaki, K.; Hirai, M.Y.; Saito, K. PRIMe Update: Innovative content for plant metabolomics and integration of gene expression and metabolite accumulation. Plant Cell Physiol. 2013, 54. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Hochmuth, D. Terpenoids Library: Terpenoids and related constituents of essential oils. Available online: http://massfinder.com/wiki/Terpenoids_Library (accessed on 4 December 2014).
- Skogerson, K.; Wohlgemuth, G.; Barupal, D.K.; Fiehn, O. The volatile compound BinBase mass spectral database. BMC Bioinform. 2011, 12. [Google Scholar] [CrossRef]
- Davik, J.; Koehler, G.; From, B.; Torp, T.; Rohloff, J.; Eidem, P.; Wilson, R.C.; Sonsteby, A.; Randall, S.K.; Alsheikh, M. Dehydrin, alcohol dehydrogenase, and central metabolite levels are associated with cold tolerance in diploid strawberry (Fragaria spp.). Planta 2013, 237, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Dhuli, P.; Rohloff, J.; Strimbeck, G.R. Metabolite changes in conifer buds and needles during forced bud break in Norway spruce (Picea abies) and European silverfir (Abies alba). Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohloff, J.; Kopka, J.; Erban, A.; Winge, P.; Wilson, R.C.; Bones, A.M.; Davik, J.; Randall, S.K.; Alsheikh, M.K. Metabolite profiling reveals novel multi-level cold responses in the diploid model Fragaria vesca (woodland strawberry). Phytochemistry 2012, 77, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; McCallum, J. Chemistry of flavonoids. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value, and Stability; De la Rosa, L.A., Alvarez-Parrilla, E., González-Aguilar, G.A., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010. [Google Scholar]
- Rohloff, J. Impact of agricultural and environmental factors on strawberry (Fragaria x ananassa Duch.) aroma—A review. Eur. J. Plant Sci. Biotechnol. 2011, 5, 17–34. [Google Scholar]
- Ellis, C.L.; Edirisinghe, I.; Kappagoda, T.; Burton-Freeman, B. Attenuation of meal-induced inflammatory and thrombotic responses in overweight men and women after 6-week daily strawberry (Fragaria) intake. A randomized placebo-controlled trial. J. Atheroscler. Thromb. 2011, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J.; Parelman, M.A.; Freytag, T.L.; Stephensen, C.B.; Kelley, D.S.; Mackey, B.E.; Woodhouse, L.R.; Bonnel, E.L. Effects of dietary strawberry powder on blood lipids and inflammatory markers in obese human subjects. Br. J. Nutr. 2012, 108, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk, I.; Lewik-Tsirigotis, M.; Capek, P.; Matulova, M.; Sasinkova, V.; Dabrowski, P.; Witkiewicz, W.; Gancarz, R. Effects of extraction condition on structural features and anticoagulant activity of F. vesca L. conjugates. Carbohydr. polym. 2013, 92, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Rogachev, I.; Kokko, H.; Mintz-Oron, S.; Venger, I.; Karenlampi, S.; Aharoni, A. Non-targeted analysis of spatial metabolite composition in strawberry (Fragariaxananassa) flowers. Phytochemistry 2008, 69, 2463–2481. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Wojdylo, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Soininen, P.; Anttonen, M.J.; Kokko, H.; Rogachev, I.; Aharoni, A.; Laatikainen, R.; Karenlampi, S. NMR and UPLC-qTOF-MS/MS characterisation of novel phenylethanol derivatives of phenylpropanoid glucosides from the leaves of strawberry (Fragaria x ananassa cv. Jonsok). Phytochem. Anal. PCA 2009, 20, 353–364. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. chiloensis form chiloensis) using HPLC-DAD-ESI-MS and free radical quenching techniques. J. Food Compos. Anal. 2010, 23, 545–553. [Google Scholar]
- Hoffmann, T.; Friedlhuber, R.; Steinhauser, C.; Tittel, I.; Skowranek, K.; Schwab, W.; Fischer, T.C. Histochemical screening, metabolite profiling and expression analysis reveal Rosaceae roots as the site of flavan-3-ol biosynthesis. Plant Biol. 2012, 14, 33–40. [Google Scholar] [PubMed]
- Rohloff, J.; Eidem, P.; Davik, J.; Alsheikh, M. Metabolic cold acclimation of ‘Polka’ and ‘Honeoye’ strawberries under natural field conditions. Acta Hortic. 2014, 1049, 463–466. [Google Scholar]
- Chikwati, E.M.; Venold, F.F.; Penn, M.H.; Rohloff, J.; Refstie, S.; Guttvik, A.; Hillestad, M.; Krogdahl, A. Interaction of soyasaponins with plant ingredients in diets for Atlantic salmon, Salmo salar L. Br. J. Nutr. 2012, 107, 1570–1590. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Ereifej, K.; Brewer, S.; Alli, I. Phenolic-protein interactions in oilseed protein isolates. Food Res. Int. 2013, 52, 178–184. [Google Scholar] [CrossRef]
- Zilic, S.; Akillioglu, H.G.; Serpen, A.; Peric, V.; Gokmen, V. Comparisons of phenolic compounds, isoflavones, antioxidant capacity and oxidative enzymes in yellow and black soybeans seed coat and dehulled bean. Eur. Food Res. Technol. 2013, 237, 409–418. [Google Scholar] [CrossRef]
- Karamac, M.; Kosinska, A.; Estrella, I.; Hernandez, T.; Duenas, M. Antioxidant activity of phenolic compounds identified in sunflower seeds. Eur. Food Res. Technol. 2012, 235, 221–230. [Google Scholar] [CrossRef]
- Shchekoldina, T.; Aider, M. Production of low chlorogenic and caffeic acid containing sunflower meal protein isolate and its use in functional wheat bread making. J. Food Sci. Technol. 2014, 51, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Maksimović Dragišić, J.; Maksimović, V.; Maksimović, M.; Basić, Z.; Crevar, M.; Stanković, G. The content of antioxidants in sunflower seed and kernel. Helia 2010, 33, 75–84. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Bjorck, I.; Ostman, E.; Kristensen, M.; Anson, N.M.; Price, R.K.; Haenen, G.R.M.M.; Havenaar, R.; Knudsen, K.E.B.; Frid, A.; Mykkanen, H.; et al. Cereal grains for nutrition and health benefits: Overview of results from in vitro, animal and human studies in the HEALTHGRAIN project. Trends Food Sci. Technol. 2012, 25, 87–100. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Phenolic compounds in cereal grains and their health benefits. Cereal Food World 2007, 52, 105–111. [Google Scholar]
- Ward, J.L.; Poutanen, K.; Gebruers, K.; Piironen, V.; Lampi, A.M.; Nystrom, L.; Andersson, A.A.; Aman, P.; Boros, D.; Rakszegi, M.; et al. The HEALTHGRAIN Cereal Diversity Screen: Concept, results, and prospects. J. Agric. Food Chem. 2008, 56, 9699–9709. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Pihlava, J.M.; Hellstrom, J. Contents of phenolic acids, alkyl- and alkenylresorcinols, and avenanthramides in commercial grain products. J. Agric. Food Chem. 2005, 53, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C.; Kamal-Eldin, A.; Marklund, M.; Andersson, A.; Aman, P.; Landberg, R. Alkylresorcinols in Swedish cereal food products. J. Food Compos. Anal. 2012, 28, 119–125. [Google Scholar] [CrossRef]
- Ross, A.B.; Shepherd, M.J.; Schupphaus, M.; Sinclair, V.; Alfaro, B.; Kamal-Eldin, A.; Aman, P. Alkylresorcinols in cereals and cereal products. J. Agric. Food Chem. 2003, 51, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Landberg, R.; Kamal-Eldin, A.; Andersson, A.; Vessby, B.; Aman, P. Alkylresorcinols as biomarkers of whole-grain wheat and rye intake: Plasma concentration and intake estimated from dietary records. Am. J. Clin. Nutr. 2008, 87, 832–838. [Google Scholar] [PubMed]
- EFSA. Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- Baiano, A.; Gambacorta, G.; Terracone, C.; Previtali, M.A.; Lamacchia, C.; la Notte, E. Changes in phenolic content and antioxidant activity of Italian extra-virgin olive oils during storage. J. Food Sci. 2009, 74, C177–C183. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Gallina-Toschi, T.; Fernandez-Gutierrez, A. Analytical determination of polyphenols in olive oils. J. Sep. Sci. 2005, 28, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed]
- Tasioula-Margari, M.; Okogeri, O. Isolation and characterization of virgin olive oil phenolic compounds by HPLC/UV and GC-MS. J. Food Sci. 2001, 66, 530–534. [Google Scholar] [CrossRef]
- Rojas, L.B.; Quideau, S.; Pardon, P.; Charrouf, Z. Colorimetric evaluation of phenolic content and GC-MS characterization of phenolic composition of alimentary and cosmetic argan oil and press cake. J. Agric. Food Chem. 2005, 53, 9122–9127. [Google Scholar] [CrossRef] [PubMed]
- Saitta, M.; lo Curto, S.; Salvo, F.; di Bella, G.; Dugo, G. Gas chromatographic-tandem mass spectrometric identification of phenolic compounds in Sicilian olive oils. Anal. Chim. Acta 2002, 466, 335–344. [Google Scholar] [CrossRef]
- Kumari, S.; Stevens, D.; Kind, T.; Denkert, C.; Fiehn, O. Applying in-silico retention index and mass spectra matching for identification of unknown metabolites in accurate mass GC-TOF mass spectrometry. Anal. Chem. 2011, 83, 5895–5902. [Google Scholar] [CrossRef] [PubMed]
- Menikarachchi, L.C.; Hill, D.W.; Hamdalla, M.A.; Mandoiu, II; Grant, D.F. In silico enzymatic synthesis of a 400,000 compound biochemical database for nontargeted metabolomics. J. Chem. Inf. Model. 2013, 53, 2483–2492. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohloff, J. Analysis of Phenolic and Cyclic Compounds in Plants Using Derivatization Techniques in Combination with GC-MS-Based Metabolite Profiling. Molecules 2015, 20, 3431-3462. https://doi.org/10.3390/molecules20023431
Rohloff J. Analysis of Phenolic and Cyclic Compounds in Plants Using Derivatization Techniques in Combination with GC-MS-Based Metabolite Profiling. Molecules. 2015; 20(2):3431-3462. https://doi.org/10.3390/molecules20023431
Chicago/Turabian StyleRohloff, Jens. 2015. "Analysis of Phenolic and Cyclic Compounds in Plants Using Derivatization Techniques in Combination with GC-MS-Based Metabolite Profiling" Molecules 20, no. 2: 3431-3462. https://doi.org/10.3390/molecules20023431
APA StyleRohloff, J. (2015). Analysis of Phenolic and Cyclic Compounds in Plants Using Derivatization Techniques in Combination with GC-MS-Based Metabolite Profiling. Molecules, 20(2), 3431-3462. https://doi.org/10.3390/molecules20023431





