Eliminating Aluminum Toxicity in an Acid Sulfate Soil for Rice Cultivation Using Plant Growth Promoting Bacteria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Laboratory Study
| Strains | IAA (mg·L−1) | Production of Organic Acid (mg·L−1) | BNF | P solubilization from PR | |||
|---|---|---|---|---|---|---|---|
| OA | MA | SA | PA | ||||
| Bacillus sp. (PSB16) | 6.78 | 0.03 | 0.07 | 0.24 | 0.006 | +ve | From soil * 86% |
| Stenotrophomonasmaltophila (Sb16) | 55.00 | 0.06 | 0.04 | 0.39 | 0.008 | ** 62 kg·ha−1 | - |
| Burkholderiathailandensis (ASB7) | 13.16 | 0.02 | 0.05 | 0.24 | 0.012 | +ve | From broth culture (72 h) 3.4% |
| Burkholderiaseminalis (ASB21) | 12.16 | 0.09 | 0.08 | 0.42 | 0.018 | +ve | From broth culture (72 h) 2.72% |
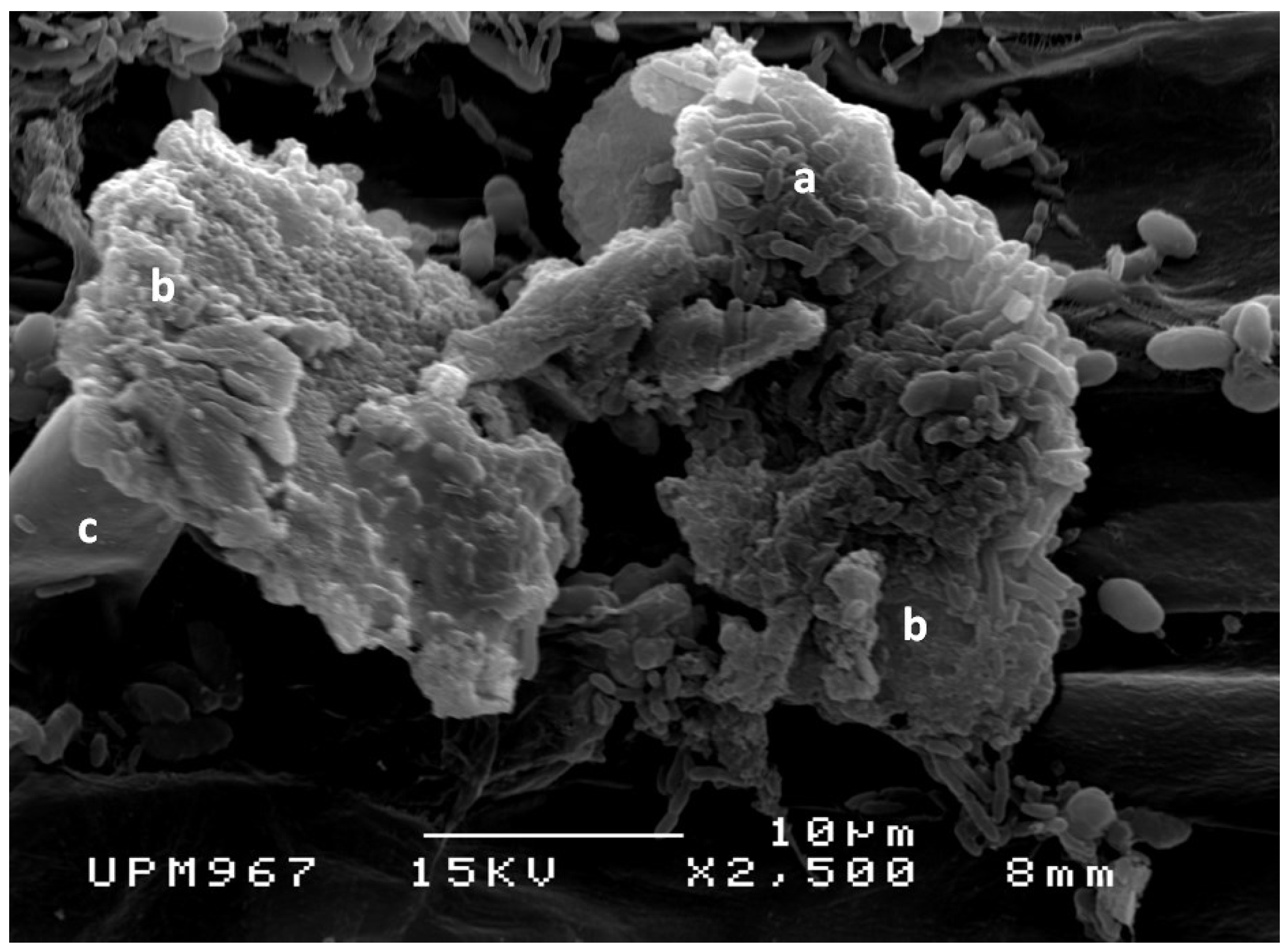
2.1.1. Effect of Al on the Growth of Rice Seedlings Inoculated with PGPB

2.1.2. Effects of Al on the Population of PGPB
| Treatments | Bacterial Population (log10CFU·mL−1) | pH Values * | ||||
|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 0 | 50 | 100 | |
| Al (μM) | ||||||
| Control | - | - | - | 3.95c | 3.25b | 2.93c |
| Bacillus sp. (PSB16) | 10.83b | 9.23b | 8.23b | 7.12a | 6.85a | 6.50a |
| Burkholderia thailandensis (ASB7) | 10.79b | 9.68a | 7.69c | 6.85b | 6.72a | 6.64a |
| Burkholderia seminalis (ASB21) | 10.87b | 9.57a | 8.11b | 6.87b | 6.65a | 6.00b |
| Stenotrophomonas maltophila (Sb16) | 11.07a | 9.61a | 8.54a | 7.09a | 6.71a | 6.30a |
2.1.3. Effects of PGPB on pH of the Growth Medium
2.1.4. Effects of Al on the Release of Organic Acids

2.1.5. Effect of Al on the Rice Roots and Leaf Cells

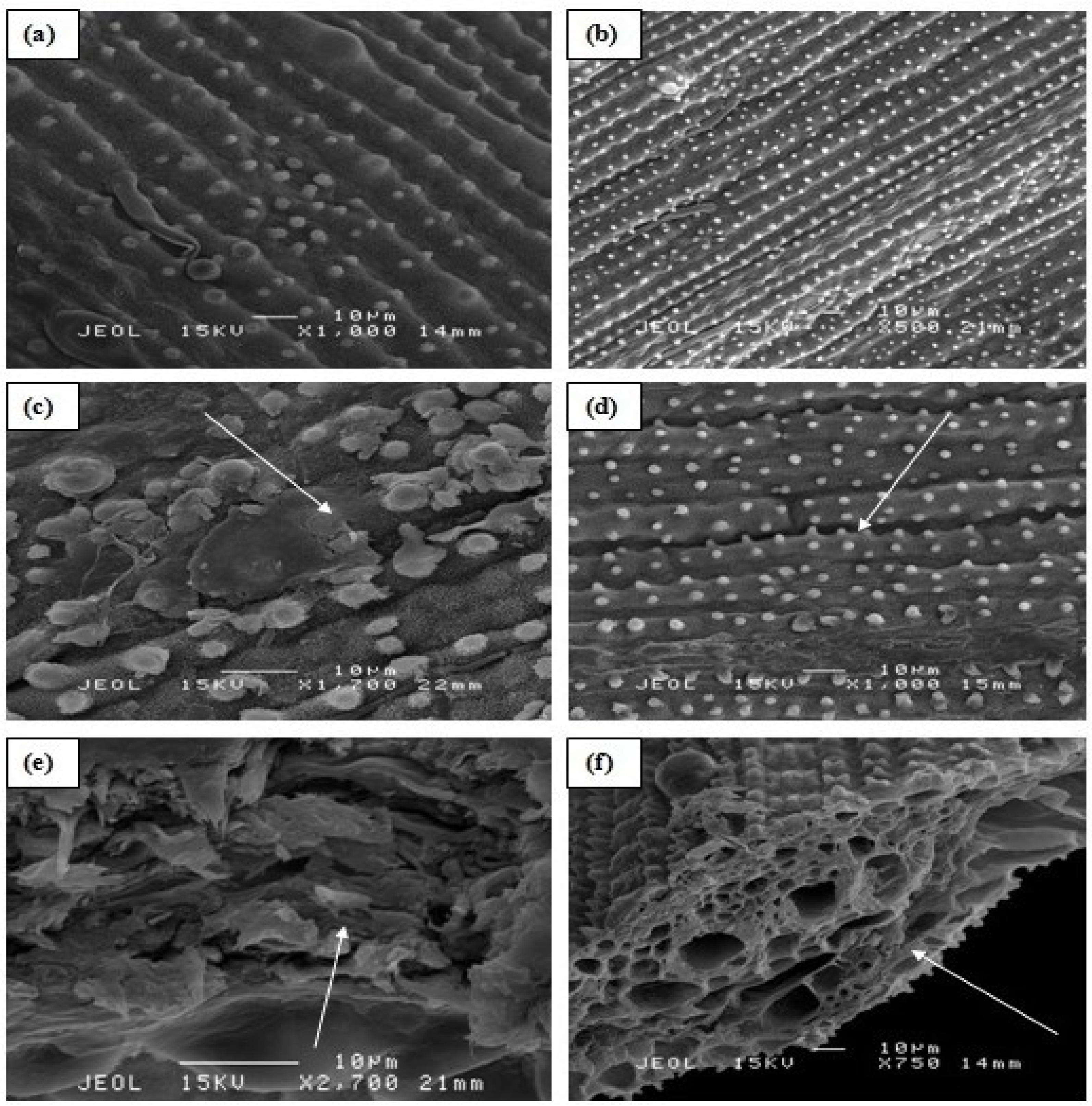
2.2. Field Study
2.2.1. Effects of Biofertilizer, Basalt and Ground Magnesium Limestone (GML) Applications on the Soil pH in the Rice Field
| Treatments | Soil pH | ||||
|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 110 | |
| Days after Sowing | |||||
| Control | 3.8a | 3.56f | 3.89d | 3.95d | 3.91d |
| Biofertilizer | 3.8a | 4.11d | 4.00c | 4.14c | 4.17c |
| a GML | 3.8a | 4.90b | 4.39a | 4.53b | 4.55ab |
| Basalt | 3.8a | 3.97e | 4.10b | 4.46b | 4.49b |
| Biofertilizer + GML | 3.8a | 5.27a | 4.50a | 4.76a | 4.79a |
| Biofertilizer + Basalt | 3.8a | 4.35c | 4.20b | 4.23c | 4.32c |
2.2.2. Effects of Biofertilizer, Basalt and Ground Magnesium Limestone (GML) Applications on the Growth of Rice and Yield
| Treatments | Root Length (cm) | Tillers plant−1 | Number of Panicle plant−1 | Size of Panicle −1 | Fertile Spikelets panicle−1 | Number of Unfilled Grains (%) | Weight of 1000 grain (g) | Grain Yield t·ha−1 | Harvest Index |
|---|---|---|---|---|---|---|---|---|---|
| Control | 19.66d | 9c | 7c | 17.83e | 61.01e | 26.21a | 17.02d | 2.93d | 0.40e |
| Biofertilizer | 32. 41a | 20a | 14ab | 20.10c | 119.51b | 16.12f | 21.40c | 5.39b | 0.35d |
| a GML | 21.34b | 19a | 15a | 22.60b | 116.81b | 18.31d | 22.33b | 5.36b | 0.45b |
| Basalt | 20.13c | 16b | 16a | 18.33d | 98.85d | 20.45b | 20.01c | 3.47c | 0.41c |
| Biofertilizer + basalt | 22.30b | 19a | 15a | 23.00b | 107.32c | 19.24c | 23.68a | 5.33c | 0.47b |
| Biofertilizer + GML | 32.58a | 21a | 15a | 24.23a | 129.03a | 17.82e | 22.55b | 6.82a | 0.55a |
2.2.3. Effects of Application of Amendments on the Form of Aluminum in the Soil
| Amendments (4 t·ha−1 each) | Exchangeable Al | Weakly-bound Al | Strongly-bound Al |
|---|---|---|---|
| cmolc kg−1 | |||
| Control | 2.09a | 3.04a | 4.78f |
| Biofertilizer | 0.11b | 2.03b | 11.69a |
| GML a | 0.03d | 1.13f | 7.23d |
| Basalt | 0.07c | 1.65c | 9.02b |
| Biofertilizer + GML | 0.01e | 1.34e | 6.34e |
| Biofertilizer + basalt | 0.04d | 1.42d | 8.53c |
2.3. Discussion
3. Experimental Section
3.1. Experimental Site/Preparation and Conditions
3.2. Laboratory Study
3.2.1. Preparation of Inocula and Rice Seedlings Inoculation under in vitro Condition
3.2.2. Determination of Microbial Population at Different Al Concentrations
3.2.3. Determination of Organic Acids and Indoleacetic Acid
3.2.4. Determination of Root Morphology
3.2.5. Visual Observation of Plant Leaf and Root Cells
3.3. Field Study
3.3.1. Bio-Fertilizer, GML and Basalt Application and Transplanting
3.3.2. Speciation of the Al in the Soils
3.3.3. Rice Yield and Yield Contributing Characters
3.3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GML | ground magnesium basalt |
| HPLC | high performance liquid chromatography |
| PGPB | Plant growth promoting bacteria |
| PSB | phosphate solubilizing bacteria |
| SEM | scanning electron microscope |
References
- Marschner, H. Mechanisms of adaptation of plants to acid soil. Plant Soil. 1991, 134, 1–20. [Google Scholar]
- Inostroza-Blancheteau, C.; Soto, B.; Ulloa, P.; Aquea, F. Resistance mechanisms of aluminum (Al3+) phytotoxicity in cereals: Physiological, genetic and molecular bases. J. Soil Sci. Plant Nutr. 2008, 8, 57–71. [Google Scholar]
- Ramgreeb, S.; Cook, J.A.; Watt, M.P. Responses of meristematic callus cells of two Cynodondactylon genotypes to aluminum. J. Plant Physiol. 2004, 161, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Ma, J.F.; Matsumoto, H. Aluminum-induced secretion of both citrate and malate in rye. Plant Soil. 2002, 242, 235–243. [Google Scholar] [CrossRef]
- Mossor-Pietraszewska, T. Effect of aluminum on plant growth and metabolism. Acta Biochim. Pol. 2001, 3, 367–686. [Google Scholar]
- Jones, D.L.; Ryan, P.R. (Eds.) Nutrition. Aluminum toxicity. In Encyclopedia of Applied Plant Science; Elsevier Science: London, UK, 2003; pp. 656–664.
- Kochian, L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Zheng, S.; Ma, J.F.; Matsumoto, H. High aluminum resistance in buckwheat: I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol. 1998, 117, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.M. Aluminum-induced cell wall peroxidase activity and lignin synthesis are differentially regulated by jasmonate and nitric oxide. J. Agric. Food Chem. 2008, 56, 9676–9684. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Naher, U.A.; Radziah, O.; Shamsuddin, Z.H.; Halimi, M.S.; Mohd Razi, I. Isolation of diazotrophs from different soils of Tanjong Karang rice growing area in Malaysia. Int. J. Agric. Biol. 2009, 11, 547–552. [Google Scholar]
- Panhwar, Q.A.; Radziah, O.; Zaharah, A.R.; Shariah, M.; Mohd Razi, I. Isolation and characterization of phosphorus solubilizing bacteria from Aerobic rice. Afr. J. Biotechnol. 2012, 11, 2711–2719. [Google Scholar]
- Nakkeeran, S.; Dilantha Fernando, W.G.; Siddiqui, Z.A. Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pest and diseases. In PGPR: Biocontrol and Biofertilization; Siddiqui, Z.A., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 257–296. [Google Scholar]
- Ngoc Son, T.; Diep, C.N.; Giang, T.T.M. Effect of bradyrhizobia and phosphate solubilizing bacteria application on soybean in rotational system in the mekong delta. Omonrice 2006, 14, 48–57. [Google Scholar]
- Pineros, M.A.; Kochian, L.V. Overview of the structure-function relations underlying functionality of ALMT and MATE-type transporters involved in the organic acid release Al tolerance response. In Proceedings of the 7th International Symposium on Plant-soil interactions at low pH, Guangzhou, China, 17–21 May 2009; Liao, H., Yan, X., Kochian, L.V., Eds.; South China University of Technology Press: Guangzhou, China, 2009; pp. 55–56. [Google Scholar]
- Yang, L.T.; Qi, Y.P.; Jiang, H.X.; Chen, L.S. Roles of organic acid anion secretion in aluminium tolerance of higher plants. Biomed. Res. Int. 2013, 2013, 173682. [Google Scholar] [PubMed]
- Ma, J.F.; Shen, R.; Zhou, Z.; Misawa, M.; Takeuchi, Y. Response of rice to aluminum stress and identification of quantitative trait loci for aluminum tolerance. Plant Cell Physiol. 2002, 43, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Elisa Azura, A.; Shamshuddin, J.; Fauziah, C.I. Root elongation, root surface area and organic acid exudation by rice seedling under Al3+ and/or H+ stress. Am. J. Agric. Biol. 2011, 6, 324–331. [Google Scholar] [CrossRef]
- Ismail, H.; Shamshuddin, J.; Syed Omar, S.R. Alleviation of soil acidity in ultisol and oxisol for corn growth. Plant Soil. 1993, 151, 55–65. [Google Scholar] [CrossRef]
- Ward, J.T.; Lahner, B.; Yakubova, E.; Salt, D.E.; Raghothama, K.G. The Effect of Iron on the Primary Root Elongation of Arabidopsis during Phosphate Deficiency. Plant Physiol. 2008, 147, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Aciego Pietri, J.C.; Brookes, P.C. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol. Biochem. 2008, 40, 1856–1861. [Google Scholar] [CrossRef]
- Bååth, E. Growth rates of bacterial communities in soils at varying pH: a comparison of the thymidine and leucine incorporation techniques. Microb. Ecol. 1998, 36, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Shamshuddin, J.; Elisa, A.A.; Shazana, M.A.R.S.; Fauziah, C.I.; Panhwar, Q.A.; Naher, U.A. Properties and management of acid sulphate soils in Southeast Asia for sustainable cultivation of rice, oil palm and cocoa. Adv. Agron. 2014, 124, 91–142. [Google Scholar]
- Li, Y.; Yang, G.X.; Luo, L.T. Aluminum sensitivity and tolerance in model and elite wheat varieties. Cereal Res. Commun. 2008, 36, 257–267. [Google Scholar] [CrossRef]
- Zheng, S.J.; Yang, J.L. Target sites of aluminum phytotoxicity. Biol. Plant. 2005, 49, 321–331. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zhang, Y.; Zheng, S. Possible involvement of cell wall pectic polysaccharides in Al resistance of some plant species. In Proceedings of the 7th International Symposium on Plant-Soil Interaction at Low pH, Guangzhou, China, 17–21 May 2009; Liao, H., Yan, X., Kochian, L., Eds.; South China University of Technology Press: Guangzhou, China, 2009; pp. 57–58. [Google Scholar]
- Satish, A.B.; Damodar, V.P. Aluminum toxicity in plants—A review. J. Appl. Chem. 2013, 2, 447–474. [Google Scholar]
- Foy, C.D. Tolerance of barley cultivars to an acid, aluminum-toxic subsoil related to mineral element concentrations in their shoots. J. Plant Nutr. 1996, 19, 1361–1380. [Google Scholar] [CrossRef]
- Farhana, J.A.; Shamshuddin, J.; Fauziah, C.I.; Husni, M.H.A. Effects of Al3+, Fe+3 and H+ on root elongation and root surface area of rice seedling. In First National LRGS Rice Research Colloquium; UPM Press: Serdang, Malaysia, 2013; p. 45. [Google Scholar]
- Kopittke, P.M.; Blamey, F.P.C.; Menzies, N.W. Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant Soil 2008, 303, 217–227. [Google Scholar] [CrossRef]
- Liao, H.; Wan, H.; Shaff, J.; Wang, X.; Yan, X.; Kochian, L.V. Phosphorus and Aluminum interaction in soybean in relation to Al tolerance. Exudation of specific organic acids from different region of the intact root system. Plant Physiol. 2006, 141, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Zhou, M.; Sun, D.; Li, C. Molecular approaches unravel the mechanism of acid soil tolerance in plants. Crop J. 2013, 1, 91–104. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition, 1st ed.; CRC/Taylor and Francis: London, UK, 2007; p. 613. [Google Scholar]
- Sunil Pawar, T.; Amarsinh Bhosale, A.; Trishala Gawade, B.; TejswiniNale, R. Isolation, screening and optimization of exopolysaccharide producing bacterium from saline soil. J. Microbiol. Biotechnol. Res. 2013, 3, 24–31. [Google Scholar]
- Caiola, M.G.; Billi, D.; Friedmann, E.I. Effect of desiccation on envelopes of the cyanobacterium Chroococcidiopsis sp. (Chroococcales). Eur. J. Physiol. 1996, 31, 97–105. [Google Scholar]
- Kaplan, D.; Christiaen, D.; Arad, S.M. Chelating Properties of Extracellular Polysaccharides from Chlorella spp. Appl. Environ. Microbiol. 1987, 53, 953–2956. [Google Scholar]
- Ridolfi, M.; Garrec, J.P. Consequences of the excess al and a deficiency in ca and mg for stomatal functioning and net carbon assimilation of beech leaves. Annu. Sci. 2000, 57, 209–218. [Google Scholar] [CrossRef]
- Bottini, R.; Cassán, F.; Piccoli, P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004, 65, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Bloemberg, G.V.; Lugtenberg, B.J. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 2011, 4, 343–350. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Shamshuddin, J.; Naher, U.A.; Radziah, O.; Mohd Razi, I. Biochemical and molecular characterization of potential phosphate-solubilizing bacteria in acid sulfate soils and their beneficial effects on rice growth. PLoS One 2014, 9, e97241. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, Q.A.; Shamshuddin, J.; Naher, U.A.; Radziah, O.; Mohd Razi, I. Changes in the chemical properties of an acid sulfate soil and the growth of rice as affected by bio-fertilizer, ground magnesium limestone and basalt application. Pedosphere 2014, 24, 827–835. [Google Scholar] [CrossRef]
- Somasegaran, P.; Hoben, H.J. Methods in Legume-Rhizobium Technology; University of Hawaii NifTAL Project and MIRCEN, Department of Agronomy and Soil Science, Hawaii Institute of Tropical Agriculture and Human Resources, College of Tropical Agriculture and Human Resources, 1985; p. 365. [Google Scholar]
- Amin, M.A.; Uddin, M.A.; Hossain, A. Regeneration study of some Indica rice cultivars followed by Agrobacterium-Mediated transformation of highly regenerable cultivar BR-8. J. Biol. Sci. 2004, 4, 207–211. [Google Scholar] [CrossRef]
- Gordon, A.S.; Weber, R.P. Colorometric estimation of indoleacetic acid. Plant Physiol. 1950, 26, 192–195. [Google Scholar] [CrossRef]
- Hamdy, E.L.Z.; Czarnes, S.; Hallett, P.D. Early changes in root characteristics of maize (Zea mays) following seed inoculation with the PGPR Azospirillum lipoferum CRT1. Plant Soil. 2007, 291, 109–118. [Google Scholar] [CrossRef]
- Drabek, O.; Boruvka, L.; Mladkova, L. Possible method of aluminum speciation in forest soil. J. Inorg. Biochem. 2003, 97, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Dobermann, A.; Fairhurst, T. Rice: Nutrient Disorders and Nutrient Management; International Rice Research Institute: Los Baños, Philippines, 2000; Volume 1, pp. 186–188. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panhwar, Q.A.; Naher, U.A.; Radziah, O.; Shamshuddin, J.; Razi, I.M. Eliminating Aluminum Toxicity in an Acid Sulfate Soil for Rice Cultivation Using Plant Growth Promoting Bacteria. Molecules 2015, 20, 3628-3646. https://doi.org/10.3390/molecules20033628
Panhwar QA, Naher UA, Radziah O, Shamshuddin J, Razi IM. Eliminating Aluminum Toxicity in an Acid Sulfate Soil for Rice Cultivation Using Plant Growth Promoting Bacteria. Molecules. 2015; 20(3):3628-3646. https://doi.org/10.3390/molecules20033628
Chicago/Turabian StylePanhwar, Qurban Ali, Umme Aminun Naher, Othman Radziah, Jusop Shamshuddin, and Ismail Mohd Razi. 2015. "Eliminating Aluminum Toxicity in an Acid Sulfate Soil for Rice Cultivation Using Plant Growth Promoting Bacteria" Molecules 20, no. 3: 3628-3646. https://doi.org/10.3390/molecules20033628
APA StylePanhwar, Q. A., Naher, U. A., Radziah, O., Shamshuddin, J., & Razi, I. M. (2015). Eliminating Aluminum Toxicity in an Acid Sulfate Soil for Rice Cultivation Using Plant Growth Promoting Bacteria. Molecules, 20(3), 3628-3646. https://doi.org/10.3390/molecules20033628





