Enhanced Materials from Nature: Nanocellulose from Citrus Waste
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Enzymatic Hydrolysis on the Morphology of Cellulose Fibers
2.1.1. Morphological Changes


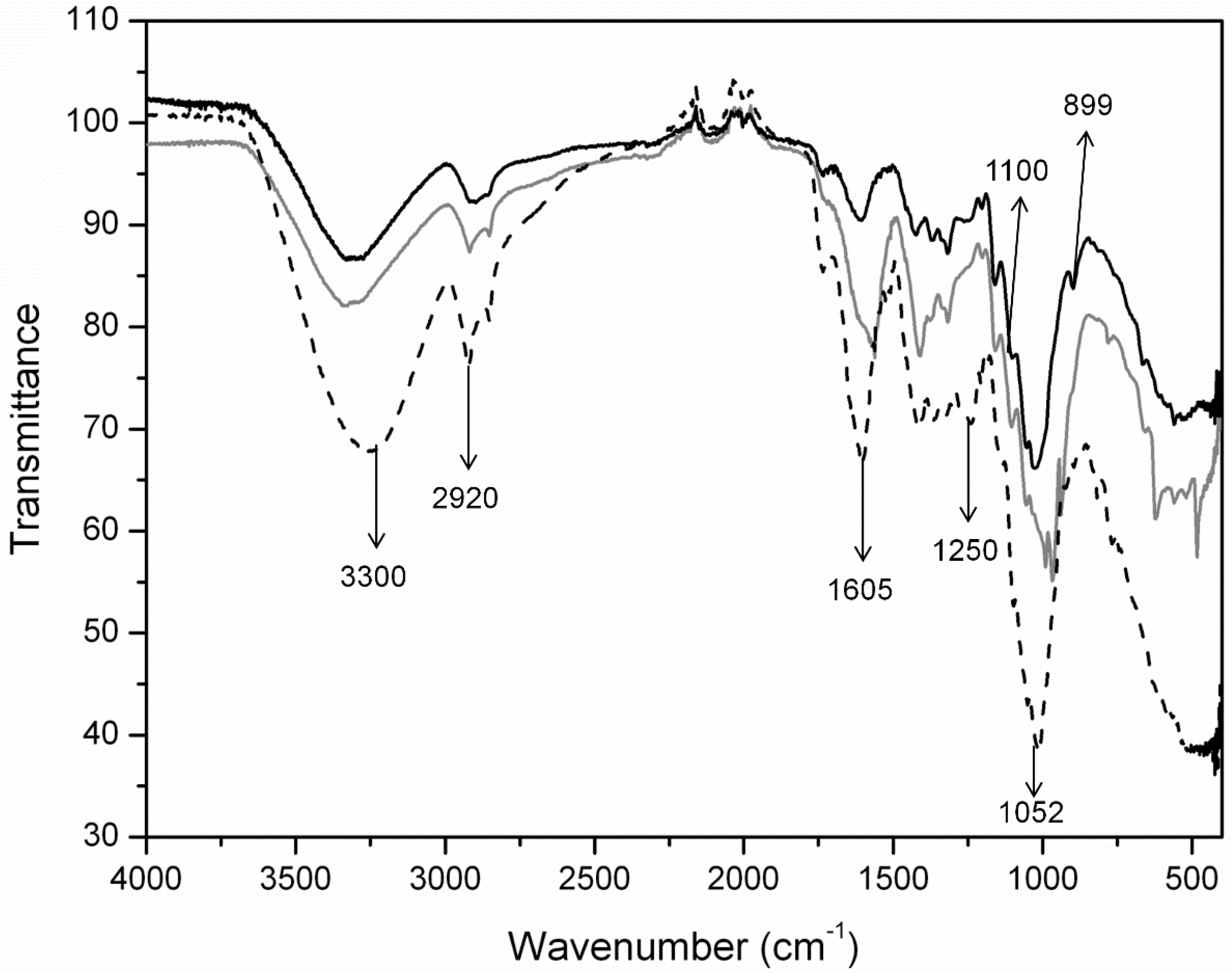
2.1.2. Structural Changes in Cellulose Fibers during the Multi-Stage Procedure
| Sample | Cellulose Content (%) | CI by XRD | CI by NMR |
|---|---|---|---|
| Raw material (CB) | 16.46 ± 0.84 | 0.16 | - |
| NaOH-treated fibers | 52.50 ± 1.35 | - | - |
| NaOH-treated and bleached fibers | 76.75 ± 0.51 | 0.50 | 0.36 |
| Chemo-enzymatic treated fibers | 83.80 ± 0.48 | 0.63 | 0.55 |


3. Experimental Section
3.1. Pretreatment of Citrus-Waste Biomass Fibers
3.2. Enzymes from Xanthomonas axonopodis pv. citri (Xac)
3.3. Enzymatic Hydrolysis and Isolation of Cellulose Nanofibers
3.4. Microstructure Analysis
3.5. FTIR Spectroscopy Analysis
3.6. NMR Spectroscopy Analysis
3.7. XRD Analyses

3.8. Cellulose Content
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thygesen, L.G.; Hidayat, B.J.; Johansen, K.S.; Felby, C. Role of supramolecular structures in enzymatic hydrolysis of plant cell walls. J. Ind. Microbiol. Biotechnol. 2011, 38, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, W.C. Fibers, fiber products, and forage fiber. In Integrative Plant Anatomy; Elsevier Science Publisher: Orlando, FL, USA, 2000; Volume 1, p. 399. [Google Scholar]
- Changsarn, S.; Mendez, J.D.; Shanmuganathan, E.; Foster, J.; Weder, C.; Supaphol, P. Biologically inspired hierarchical design of nanocomposites based on poly (ethylene oxide) and cellulose nanofibers. Macromol. Rapid. Commun. 2011, 32, 1367–1372. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Lemes, A.P.; Seabra, A.B. Review of cellulose nanocrystals patents: Preparation, composites and general applications. Recent Pat. Nanotechnol. 2012, 6, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, C.W.; Schellhorn, H.E.; Gibbons, L.N.; Maine, F.; Mason, E. The release of fermentable carbohydrate from peat by steam explosion and its use in the microbial-production of solvents. Biotechnol. Bioeng. 1986, 28, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishman, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A Review. Enzym. Res. 2011, 2011. [Google Scholar] [CrossRef]
- Chanzy, H. Cellulose Sources and Exploitation, Aspects of Cellulose Structure; Kennedy, J.F., Phillips, G.O., Williams, P.A., Eds.; Ellis Horwood Ltd.: New York, NY, USA, 1990; pp. 3–12. [Google Scholar]
- Meng, X.; Ragauskas, A.J. Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr. Opin. Biotechnol. 2014, 27, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Chang, F.; Inoue, S.; Endo, T. Increase in enzyme accessibility by generation of nanospace in cell wall supramolecular structure. Bioresour. Technol. 2010, 101, 7218–7223. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Bansal, P.; Lee, J.H.; Realff, M.J.; Bommarius, A.S. Cellulose crystallinity—A key predictor of the enzymatic hydrolysis rate. FEBS J. 2010, 277, 1571–1582. [Google Scholar] [CrossRef] [PubMed]
- Satyamurthy, P.; Jain, P.; Balasubramanya, R.H.; Vigneshwaran, N. Preparation and characterization of cellulose nanowhiskers from cotton fibres by controlled microbial hydrolysis. Carbohydr. Polym. 2011, 83, 122–129. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of nanocellulose fibrils from lignocellulose fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1486–1475. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Nazrul Islam, M.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical process: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Oskman, K.; Etang, J.A.; Mathew, A.P.; Jonoobi, M. Cellulose nanowhiskers separated from a bio-residue from wood bioethanol production. Biomass Bioenergy 2011, 35, 146–152. [Google Scholar] [CrossRef]
- Tsukamoto, J.; Durán, N.; Tasic, L. Nanocellulose and bioethanol production from orange bagasse waste using isolated microorganisms. J. Braz. Chem. Soc. 2013, 24, 1537–1543. [Google Scholar]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Preparation and characterization of long natural cellulose fibres from wheat straw. J. Agric. Food Chem. 2007, 5, 8570–8575. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Natural cellulose fibers from soybean straw. Bioresour. Technol. 2009, 100, 3593–3598. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Structural and properties of natural cellulose fibers obtained from sorghum leaves and stems. J. Agric. Food Chem. 2007, 55, 5569–5574. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, J.; Zhang, Z.; Lu, C. Study on structure and thermal stability properties of cellulose fibers from rice straw. Carbohydr. Polym. 2011, 85, 245–250. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Pasquini, D.; Teixeira, E.M.; Curvelo, A.A.S.; Belgacem, M.N.; Dufresne, A. Extraction of cellulose whiskers from cassava bagasse and their applications as reinforced agent in natural rubber. Ind. Crop. Prod. 2010, 32, 486–490. [Google Scholar] [CrossRef]
- Zuluaga, R.; Putaux, J.L.; Cruz, J.; Vélez, J.; Mondragon, I.; Gañán, P. Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features. Carbohydr. Polym. 2009, 76, 51–59. [Google Scholar] [CrossRef]
- Elanthikkal, S.; Gopalakrishnapanicker, U.; Varghese, S.; Guthrie, J.T. Cellulose microfibres produced from banana plant wastes: Isolation and characterization. Carbohydr. Polym. 2010, 80, 852–859. [Google Scholar] [CrossRef]
- Deepa, B.; Eldho, A.; Cherian, B.M.; Bismarck, A.; Blaker, J.; Laly, A.; Pothan, L.A.; Leão, A.L.; Souza, S.F.; Kottaisamy, M. Structure, morphology and thermal characteristics of banana nanofibers obtained by steam explosion. Biosour. Technol. 2011, 102, 1988–1997. [Google Scholar] [CrossRef]
- Cherian, B.M.; Pothan, L.A.; Nguyen-Chung, T.; Mennig, G.; Kottaisamy, M.; Thomas, S. A novel method for the synthesis of cellulose nanofibril whiskers from banana fibers and characterization. J. Agric. Food Chem. 2008, 56, 5617–5627. [Google Scholar] [CrossRef] [PubMed]
- Cherian, B.M.; Leão, A.L.; Souza, S.F.; Thomas, S.; Pothan, L.A.; Kottaisamy, M. Isolation of nanocellulose from pineapple leaf fibres by ateam explosion. Carbohydr. Polym. 2010, 81, 720–725. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Germinario, L.T.; Winter, W.T. Isolation, preparation and characterization of cellulose microfibers obtained from bagasse. Carbohydr. Polym. 2008, 73, 371–377. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Campos, A.; Correa, A.C.; Cannella, D.; Teixeira, E.M.; Marconcini, J.M.; Dufresne, A.; Mattoso, L.C.; Cassland, C.; Sanadi, A. Obtaining nanofibers from curauá and sugarcane bagasse fibers using enzymatic hydrolysis followed by sonication. Cellulose 2013, 20, 1491–1500. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Structure and properties of high quality natural cellulose fibers from cornstalks. Polymer 2005, 46, 5494–5500. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Properties and potential applications of natural cellulose fibers from cornhusks. Green Chem. 2005, 7, 190–195. [Google Scholar] [CrossRef]
- Haafiz, M.K.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Hsieh, Y.-L. Cellulose isolation and core-shell nanostructures of cellulose nanocrystals from chardonnay grape skins. Carbohydr. Polym. 2012, 87, 2546–2553. [Google Scholar] [CrossRef]
- Ejikeme, P.M. Investigation of the physicochemical properties of microcrystalline cellulose from agricultural wastes I: Orange mesocarp. Cellulose 2008, 15, 141–147. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguz, J.M. Submerged citric acid fermentation on orange peel autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Awan, A.T.; Tsukamoto, J.; Tasic, L. Orange waste as a biomass for 2G-ethanol production using low cost enzymes and co-culture fermentation. RSC Adv. 2013, 3, 25071–25078. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Oksman, K.; Mathew, A.P.; Bondeson, D.; Kvien, I. Manufacturing process of cellulose whiskers/polylactic acid nanocomposites. Compos. Sci. Technol. 2006, 66, 2776–2784. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to celulosecrystallinity and Crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in cellulose I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Fang, J.M.; Sun, R.C.; Tomkinson, J. Isolation and characterization of hemicelluloses and cellulose from rye straw by alkaline peroxide extraction. Cellulose 2000, 7, 87–107. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulose performing. Biotechnol. Fuels 2010, 3, 2–10. [Google Scholar]
- Segal, L.; Creely, J.; Martin, A.; Conrad, C. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1962, 29, 786–794. [Google Scholar] [CrossRef]
- Vanderhart, D.L.; Atalla, R.H. Studies of microstructure in native celluloses using solid-state 13C-NMR. Macromolecules 1984, 17, 1465–1472. [Google Scholar] [CrossRef]
- Synytsya, A.; Copíková, J.; Brus, J. 13C CP/MAS NMR spectra of pectins: A peak-fitting analysis in the C-6 region. Czech J. Food Sci. 2003, 21, 1–12. [Google Scholar]
- Mazeau, K.; Heux, L. Molecular dynamics simulations of bulk native crystalline and amorphous structures of cellulose. J. Phys. Chem. B 2003, 107, 2394–2403. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the nanocellulose obtained by both, chemical and enzymatic, treatments are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariño, M.; Lopes da Silva, L.; Durán, N.; Tasic, L. Enhanced Materials from Nature: Nanocellulose from Citrus Waste. Molecules 2015, 20, 5908-5923. https://doi.org/10.3390/molecules20045908
Mariño M, Lopes da Silva L, Durán N, Tasic L. Enhanced Materials from Nature: Nanocellulose from Citrus Waste. Molecules. 2015; 20(4):5908-5923. https://doi.org/10.3390/molecules20045908
Chicago/Turabian StyleMariño, Mayra, Lucimara Lopes da Silva, Nelson Durán, and Ljubica Tasic. 2015. "Enhanced Materials from Nature: Nanocellulose from Citrus Waste" Molecules 20, no. 4: 5908-5923. https://doi.org/10.3390/molecules20045908







