Exploiting Protected Maleimides to Modify Oligonucleotides, Peptides and Peptide Nucleic Acids
Abstract
:1. Introduction. Maleimide-Involving Click Conjugation Reactions with Oligonucleotides and Polyamides



2. Development of a Maleimide Protection Strategy Allowing Maleimido-Oligonucleotides to Be On-Resin Assembled
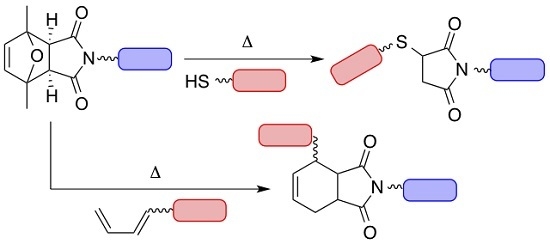
2.1. Identification of a Maleimide Protecting Group and Proof of Principle Experiments




2.2. Experiments Broadening the Scope of Applications of the Method in the Field of Oligonucleotide Conjugates



3. Further Applications of Protected Maleimides
3.1. Derivatization of Polyamides. Simultaneous Maleimide Deprotection and Conjugation Allows for Double Conjugation of Polyamides as Well as Synthesis of Oligonucleotide Conjugates



3.2. Use of the Maleimide-Thiol Reaction for Cyclization
3.2.1. Cyclic Oligonucleotides

3.2.2. Cyclic and Bicyclic Peptides

3.2.3. Conjugates of Cyclic Peptides and Peptoids

4. Use of Maleimides for Conjugation: Balance and Perspectives

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2005–2021. [Google Scholar] [CrossRef]
- Ornes, S. Antibody-drug conjugates. Proc. Natl. Acad. Sci. USA 2013, 110, 13695. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.-H.; Rudolph, M.J.; Stein, S. Preparation of oligonucleotide-peptide conjugates. Bioconjugate Chem. 1991, 2, 464–465. [Google Scholar] [CrossRef]
- Bongartz, J.-P.; Aubertin, A.-M.; Milhaud, P.G.; Lebleu, B. Improved biological activity of antisense oligonucleotides conjugated to a fusogenic peptide. Nucleic Acids Res. 1994, 22, 4681–4688. [Google Scholar] [CrossRef] [PubMed]
- Zanta, M.A.; Belguise-Valladier, P.; Behr, J.P. Gene delivery: A single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc. Natl. Acad. Sci. USA 1999, 96, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.R.; Chaput, J.C. Synthesis of peptide-oligonucleotide conjugates using a heterobifunctional crosslinker. Curr. Protoc. Nucleic. Acid Chem. 2010. [Google Scholar] [CrossRef]
- Kappe, C.O.; Murphree, S.S.; Padwa, A. Synthetic applications of furan Diels-Alder chemistry. Tetrahedron 1997, 53, 14179–14233. [Google Scholar] [CrossRef]
- Conley, N.R.; Hung, R.J.; Willson, C.G. A new synthetic route to authentic N-substituted aminomaleimides. J. Org. Chem. 2005, 70, 4553–4555. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Weber, R.; Twieg, R.J. Improved synthesis of DCDHF fluorophores with maleimide functional groups. Tetrahedron Lett. 2006, 47, 7213–7217. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Pedroso, E.; Grandas, A. Maleimide-dimethylfuran exo adducts: Effective maleimide protection in the synthesis of oligonucleotide conjugates. Org. Lett. 2011, 13, 4364–4367. [Google Scholar] [CrossRef] [PubMed]
- Kwart, H.; King, K. The reverse Diels-Alder or retrodiene reaction. Chem. Rev. 1968, 68, 415–447. [Google Scholar] [CrossRef]
- Kwart, H.; Burchuk, I. Isomerism and adduct stability in the Diels-Alder reaction. 1aI. The adducts of furan and maleimide. J. Am. Chem. Soc. 1952, 74, 3094–3097. [Google Scholar] [CrossRef]
- Sánchez, A.; Pedroso, E.; Grandas, A. Easy introduction of maleimides at different positions of oligonucleotide chains for conjugation purposes. Org. Biomol. Chem. 2012, 10, 8478–8483. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Kim, S.; Chiba, K. Synthesis of conjugated oligonucleotide in solution phase using alkyl-chain-soluble support. Chem. Lett. 2014, 43, 1251–1253. [Google Scholar] [CrossRef]
- Sánchez, A.; Pedroso, E.; Grandas, A. Conjugation reactions involving maleimides and phosphorothioate oligonucleotides. Bioconjugate Chem. 2012, 23, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Elduque, X.; Sánchez, A.; Sharma, K.; Pedroso, E.; Grandas, A. Protected maleimide building blocks for the decoration of peptides, peptoids, and peptide nucleic acids. Bioconjugate Chem. 2013, 24, 832–839. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.K.; Wu, A.D.; Chandramouli, N. Maleimide-assisted on-resin macrocyclization. Tetrahedron Lett. 1996, 37, 5665–5668. [Google Scholar] [CrossRef]
- Sánchez, A.; Pedroso, E.; Grandas, A. Oligonucleotide cyclization: The thiol-maleimide reaction revisited. Chem. Commun. 2013, 49, 309–311. [Google Scholar] [CrossRef]
- Pal, B.; Pradhan, P.K.; Jaisankar, P.; Gir, V.S. First triphenylphosphine promoted reduction of maleimides to succinimides. Synthesis 2003, 10, 1549–1552. [Google Scholar]
- Elduque, X.; Pedroso, E.; Grandas, A. Straightforward synthesis of cyclic and bicyclic peptides. Org. Lett. 2013, 15, 2038–2041. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Albericio, F.; Solé, N.A.; Munson, M.C.; Ferrer, M.; Barany, G. Formation of disulfide bonds in synthetic peptides and proteins. In Peptide Synthesis Protocols: Methods in Molecular Biology; Pennington, M.W., Dunn, B.M., Eds.; Humana Press: Totowa, NJ, USA, 1994; Volume 35, pp. 91–169. [Google Scholar]
- Elduque, X.; Pedroso, E.; Grandas, A. Orthogonal protection of peptides and peptoids for cyclization by the thiol-ene reaction and conjugation. J. Org. Chem. 2014, 79, 2843–2853. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.D. The stability of N-ethylmaleimide and its reaction with sulfhydryl groups. J. Am. Chem. Soc. 1955, 77, 3922–3923. [Google Scholar] [CrossRef]
- Cal, P.M.S.D.; Bernardes, G.J.L.; Gois, P.M.P. Cysteine-selective reactions for antibody conjugation. Angew. Chem. Int. Ed. 2014, 53, 10585–10587. [Google Scholar] [CrossRef]
- Lewis, M.R.; Shively, J.E. Maleimidocysteinamido-DOTA derivatives: New reagents for radiometal chelate conjugation to antibody sulhydryl groups undergo pH-dependent cleavage reactions. Bioconjugate Chem. 1998, 9, 72–86. [Google Scholar] [CrossRef]
- Baldwin, A.; Kiick, K.L. Tunable degradation of maleimide-thiol adducts in reducing environments. Bioconjugate Chem. 2011, 22, 1946–1953. [Google Scholar] [CrossRef]
- Alley, S.C.; Benjamin, D.R.; Jeffrey, S.C.; Okeley, N.M.; Meyer, D.L.; Sanderson, R.J.; Senter, P.D. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjugate Chem. 2008, 19, 759–765. [Google Scholar] [CrossRef]
- Strop, P.; Liu, S.H.; Dorywalska, M.; Delaria, K.; Dushin, R.G.; Tran, T.T.; Ho, W.H.; Farias, S.; Casas, M.G.; Abdiche, Y.; et al. Location matters: Site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem. Biol. 2013, 20, 161–167. [Google Scholar]
- Shen, B.-Q.; Xu, K.; Liu, L.; Raab, H.; Bhakta, S.; Kenrich, M.; Parsons-Reponte, K.L.; Tien, J.; Yu, S.F.; Mai, E.; et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nature Biotecnol. 2012, 30, 184–189. [Google Scholar]
- Lyon, R.P.; Setter, J.R.; Bovee, T.D.; Doronina, S.O.; Hunter, J.H.; Anderson, M.E.; Balasubramanian, C.L.; Duniho, S.M.; Leiske, C.I.; Li, F.; et al. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nature Biotecnol. 2014, 32, 1059–1062. [Google Scholar]
- Tumey, L.N.; Charati, M.; He, T.; Sousa, E.; Ma, D.; Han, X.; Clark, T.; Casavant, J.; Loganzo, F.; Barletta, F.; et al. Mild method for succinimide hydrolysis on ADCs: Impact on ADC potency, stability, exposure and efficacy. Bioconjugate Chem. 2014, 25, 1871–1880. [Google Scholar]
- Portela, C.; Albericio, F.; Eritja, R.; Castedo, L.; Mascareñas, J.L. ds-Oligonucleotide-peptide conjugates featuring peptides from the leucine-zipper region of Fos as switchable receptors for the oncoprotein Jun. ChemBioChem 2007, 8, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Y.; Feng, C.; Wang, Q.; Shi, H.; Zhao, D.; Yu, R.; Su, Z. Loss of PEG chain in routine SDS-PAGE analysis of PEG-maleimide modified protein. Electrophoresis 2015, 36, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Machida, M.I.; Kanaoka, Y. Hydrolysis of N-substituted maleimides: Stability of fluorescence thiol reagents in aqueous media. Chem. Pharm. Bull. 1977, 25, 2739–2743. [Google Scholar] [CrossRef]
- Badescu, G.; Bryant, P.; Swierkosz, J.; Khayrzad, F.; Pawlisz, E.; Farys, M.; Cong, Y.; Muroni, M.; Rumpf, N.; Brocchini, S.; Godwin, A. A new stable reagent for stable thiol-specific conjugation. Bioconjugate Chem. 2014, 25, 460–469. [Google Scholar] [CrossRef]
- Maruani, A.; Alom, S.; Canavelli, P.; Lee, M.T.W.; Morgan, R.E.; Chudasama, V.; Caddick, S. A mild TCEP-based para-azidobenzyl cleavage strategy to transform reversible cysteine thiol labelling agents into irreversible conjugates. Chem. Commun. 2015, 51, 5279–5282. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paris, C.; Brun, O.; Pedroso, E.; Grandas, A. Exploiting Protected Maleimides to Modify Oligonucleotides, Peptides and Peptide Nucleic Acids. Molecules 2015, 20, 6389-6408. https://doi.org/10.3390/molecules20046389
Paris C, Brun O, Pedroso E, Grandas A. Exploiting Protected Maleimides to Modify Oligonucleotides, Peptides and Peptide Nucleic Acids. Molecules. 2015; 20(4):6389-6408. https://doi.org/10.3390/molecules20046389
Chicago/Turabian StyleParis, Clément, Omar Brun, Enrique Pedroso, and Anna Grandas. 2015. "Exploiting Protected Maleimides to Modify Oligonucleotides, Peptides and Peptide Nucleic Acids" Molecules 20, no. 4: 6389-6408. https://doi.org/10.3390/molecules20046389
APA StyleParis, C., Brun, O., Pedroso, E., & Grandas, A. (2015). Exploiting Protected Maleimides to Modify Oligonucleotides, Peptides and Peptide Nucleic Acids. Molecules, 20(4), 6389-6408. https://doi.org/10.3390/molecules20046389