A Facile Synthesis and Antimicrobial Activity Evaluation of Sydnonyl-Substituted Thiazolidine Derivatives
Abstract
:1. Introduction
2. Results and Discussion
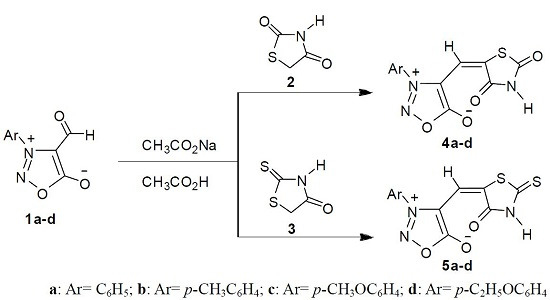
2.1. Synthetic Chemistry


| Compounds | 4b | 5a |
|---|---|---|
| Diffractometer | Nonius Kappa CCD | Nonius Kappa CCD |
| Formula | C13H9N3O4S | C12H7N3O3S2 |
| Formula weight | 303.29 | 305.33 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P2(1)/c | P2(1)/c |
| a/Å | 7.46360(10) | 16.749(3) |
| b/Å | 22.0840(5) | 4.9332(10) |
| c/Å | 8.2955(2) | 24.777(5) |
| α/° | 90.00 | 90.00 |
| β/° | 98.2900(14) | 140.16(3) |
| γ/° | 90.00 | 90.00 |
| V/Å3 | 1353.03(5) | 1311.7(5) |
| Z | 4 | 4 |
| Dcalc (g·cm−3) | 1.489 | 1.546 |
| F000 | 624.00 | 624 |
| Absorption coefficient (mm−1) | 0.259 | 0.416 |
| Crystal size/mm | 0.30 × 0.25 × 0.20 | 0.30 × 0.25 × 0.20 |
| Temperature (K) | 295(2) | 295(2) |
| θrange, deg | 1.84–27.47 | 1.65–27.49 |
| Reflections collected | 8949 | 13460 |
| Independent reflections | 3075 [ R(int) = 0.0318] | 2940 [ R(int) = 0.1322] |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Final R indices [I > 2.00σ(I)] | R1 = 0.0427, WR2 = 0.1123 | R1 = 0.0585, WR2 = 0.1584 |
| R indices (all data) | R1 = 0.0653, WR2 = 0.1339 | R1 = 0.0814, WR2 = 0.1904 |
| GoF | 1.096 | 1.052 |


2.2. Evaluation of Antimicrobial Activity
| Compounds | Aspergillus Niger * | Penicillum Citrinum * | ||
|---|---|---|---|---|
| Inhibition Zone (mm) | Relative Inhibition (%) | Inhibition Zone (mm) | Relative Inhibition (%) | |
| 4a (Ar = C6H5) | 19 | 282.88 ± 5.91 | 20 | 175.01 ± 3.81 |
| 4b (Ar = p-CH3C6H4) | 19 | 282.88 ± 6.61 | 19 | 154.70 ± 1.62 |
| 4c (Ar = p-CH3OC6H4) | 20 | 320.04 ± 9.32 | 20 | 175.07 ± 8.51 |
| 4d (Ar = p-C2H5OC6H4) | 20 | 320.06 ± 11.21 | 21 | 196.38 ± 5.05 |
| 5a (Ar = C6H5) | 19 | 282.91 ± 9.34 | 23 | 242.24 ± 6.51 |
| 5b (Ar = p-CH3C6H4) | 21 | 351.11 ± 7.93 | 23 | 242.23 ± 7.59 |
| 5c (Ar = p-CH3OC6H4) | 23 | 442.92 ± 9.39 | 24 | 266.68 ± 4.56 |
| 5d (Ar = p-C2H5OC6H4) | 23 | 442.87 ± 12.37 | 24 | 266.67 ± 2.89 |
| G (Griseofulvin) | 13 | 100 | 16 | 100 |
| B (DMF) | 8 | - | 8 | - |

3. Experimental Section
3.1. General
3.2. Syntheses of 5-(3-Arylsydnon-4-ylmethylene)thiazolidine-2,4-diones 4a–d
3.3. Syntheses of 5-(3-Arylsydnon-4-ylmethylene)-2-thioxothiazolidin-4-ones 5a–d
3.4. Biological Evaluation (Antimicrobial Activity)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ban, J.O.; Kwak, D.H.; Oh, J.H.; Park, E.J.; Cho, M.C.; Song, H.S.; Song, M.J.; Han, S.B.; Moon, D.C.; Kang, K.W.; et al. Suppression of NF-κB and GSK-3β is involved in colon cancer cell growth inhibition by the PPAR agonist troglitazone. Chem. Biol. Interact. 2010, 188, 75–85. [Google Scholar] [PubMed]
- El-Gaby, M.S.A.; Ismail, Z.H.; Abdel-Gawad, S.M.; Aly, H.M.; Ghorab, M.M. Synthesis of thiazolidine and thiophene derivatives for evaluation as anticancer agents. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 2645–2654. [Google Scholar] [CrossRef]
- Beharry, Z.; Zemskova, M.; Mahajan, S.; Zhang, F.; Ma, J.; Xia, Z.; Lilly, M.; Smith, C.D.; Kraft, A.S. Novel benzylidene-thiazolidine-2,4-diones inhibit Pim protein kinase activity and induce cell cycle arrest in leukemia and prostate cancer cells. Mol. Cancer Ther. 2009, 8, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Mosula, L.; Zimenkovsky, B.; Vasylenko, O.; Gzella, A.; Lesyk, R. Synthesis and anticancer activity evaluation of 4-thiazolidinones containing benzothiazole moiety. Eur. J. Med. Chem. 2010, 45, 5012–5021. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.W.A.; Silva, T.G.; da Rocha Pitta, M.G.; Bezerra, D.P.; Costa-Lotufo, L.V.; de Moraes, M.O.; Pessoa, C.; de Moura, M.A.F.B.; de Abreu, F.C.; de Lima, M.C.A.; et al. Synthesis and cytotoxic activity of new acridine-thiazolidine derivatives. Bioorg. Med. Chem. 2012, 20, 3533–3539. [Google Scholar] [CrossRef] [PubMed]
- Onen-Bayram, F.E.; Durmaz, I.; Scherman, D.; Herscovici, J.; Cetin-Atalay, R. A novel thiazolidine compound induces caspase-9 dependent apoptosis in cancer cells. Bioorg. Med. Chem. 2012, 20, 5094–5102. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.D.; Amato, A.A.; de Oliveira, T.B.; Iannini, K.B.R.; da Silva, A.L.; da Silva, T.G.; Leite, E.S.; Hernandes, M.Z.; de Lima, M.C.A.; Galdino, S.L.; et al. Synthesis and anti-inflammatory activity of new arylidene-thiazolidine-2,4-diones as PPARγ ligands. Bioorg. Med. Chem. 2010, 18, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Faidallah, H.M.; Khan, K.A.; Asiri, A.M. Synthesis and biological evaluation of new 3,5-di(trifluoromethyl)-1,2,4-triazolesulfonylurea and thiourea derivatives as antidiabetic and antimicrobial agents. J. Fluor. Chem. 2011, 132, 870–877. [Google Scholar] [CrossRef]
- Tomašić, T.; Kovač, A.; Simčič, M.; Blanot, D.; Grdadolnik, S.G.; Gobec, S.; Kikelj, D.; Mašič, L.P. Novel 2-thioxothiazolidin-4-one inhibitors of bacterial MurD ligase targeting D-Glu- and diphosphate-binding sites. Eur. J. Med. Chem. 2011, 46, 3964–3975. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Rajpara, K.M.; Joshi, V.V. Microwave induced synthesis of fluorobenzamides containing thiazole and thiazolidine as promising antimicrobial analogs. J. Fluor. Chem. 2013, 145, 102–111. [Google Scholar] [CrossRef]
- D’Ascenzio, M.; Bizzarri, B.; De Monte, C.; Carradori, S.; Bolasco, A.; Secci, D.; Rivanera, D.; Faulhaber, N.; Bordón, C.; Jones-Brando, L. Design, synthesis and biological characterization of thiazolidin-4-one derivatives as promising inhibitors of Toxoplasma gondii. Eur. J. Med. Chem. 2014, 17, 17–30. [Google Scholar] [CrossRef]
- E Silva, A.K.S.; Torres, D.O.C.; Rocha, S.W.S.; Gomes, F.O.S.; Silva, B.S.; Donato, M.A.M.; Raposo, C.; Santos, A.C.O.; de Lima, M.C.A.; Galdino, S.L.; et al. Effect of new thiazolidine derivatives LPSF/GQ-02 and LPSF/GQ-16 on atherosclerotic lesions in LDL receptor-deficient mice (LDLR−/−). Cardiovasc. Pathol. 2013, 22, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.W.A.; Bezerra, D.P.; Ferreira, P.M.P.; Cavalcanti, B.C.; Silva, T.G.; Pitta, M.G.R.; de Lima, M.C.A.; Galdino, S.L.; Pitta, I.R.; Costa-Lotufo, L.V.; et al. Inhibition of DNA topoisomerase I activity and induction of apoptosis by thiazacridine derivative. Toxicol. Appl. Pharmacol. 2013, 268, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kamila, S.; Biehl, E.R. Microwave-assisted synthesis of novel bis(2-thioxothiazolidin-4-one)derivatives as potential GSK-3 inhibitors. Tetrahedron Lett. 2012, 53, 3998–4003. [Google Scholar] [CrossRef]
- Kamila, S.; Ankati, H.; Harry, E.; Biehl, E.R. A facile synthesis of novel 3-(aryl/alkyl-2-ylmethyl)-2-thioxothiazolidin-4-ones using microwave heating. Tetrahedron Lett. 2012, 53, 2195–2198. [Google Scholar] [CrossRef]
- Shah, S.; Singh, B. Urea/thiourea catalyzed, solvent-free synthesis of 5-arylidenethiazolidine-2,4-diones and 5-arylidene-2-thioxothiazolidin-4-ones. Bioorg. Med. Chem. Lett. 2012, 22, 5388–5391. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.Y.; Tien, H.J.; Huang, L.Y.; Chen, M.H. Sydnone compounds. XX. The synthesis and the Schmidt reaction of 4-formyl-3-arylsydnone. J. Chin. Chem. Soc. 1983, 30, 29–37. [Google Scholar]
- Shih, M.H. Studies on the syntheses of heterocycles from 3-arylsydnone-4-carbohydroximic acid chlorides with N-arylmaleimides, [1,4]naphthoquinone and aromatic amines. Tetrahedron 2002, 58, 10437–10445. [Google Scholar] [CrossRef]
- Shih, M.H. A concise synthetic method for sydnonyl-substituted pyrazoline derivatives. Synthesis 2004, 1, 26–32. [Google Scholar] [CrossRef]
- Shih, M.H.; Ke, F.Y. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004, 12, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.H.; Yeh, M.Y.; Lee, M.J.; Su, Y.S. Efficient syntheses of 3-(3-arylsydnon-4-yl)triazole derivatives. Synthesis 2004, 17, 2877–2885. [Google Scholar] [CrossRef]
- Shih, M.H.; Wu, C.L. Efficient syntheses of thiadiazoline and thiadiazole derivatives by the cyclization of 3-aryl-4-formylsydnone thiosemicarbazones with acetic anhydride and ferric chloride. Tetrahedron 2005, 61, 10917–10925. [Google Scholar] [CrossRef]
- Shih, M.H.; Tsai, C.H.; Wang, Y.C.; Shieh, M.Y.; Lin, G.L.; Wei, C.Y. Microwave-assisted synthesis of sydnonyl-substituted imidazoles. Tetrahedron 2007, 63, 2990–2999. [Google Scholar] [CrossRef]
- Shih, M.H.; Su, Y.S.; Wu, C.L. Syntheses of aromatic substituted hydrazino-thiazole derivatives to clarify structural characterization and antioxidant activity between 3-arylsydnonyl and aryl substituted hydrazino-thiazoles. Chem. Pharm. Bull. 2007, 58, 1126–1135. [Google Scholar] [CrossRef]
- Dunkley, C.S.; Thoman, C.J. Synthesis and biological evaluation of a novel phenyl substituted sydnone series as potential antitumor agents. Bioorg. Med. Chem. Lett. 2003, 13, 2899–2901. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.S.; Kalluraya, B.; Lingappa, B.; Shenoy, S.; Puranic, V.G. Convenient access to 1,3,4-trisubstituted pyrazoles carrying 5-nitrothiophene moiety via 1,3-dipolar cycloaddition of sydnones with acetylenic ketones and their antimicrobial evaluation. Eur. J. Med. Chem. 2008, 43, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Taj, T.; Kamble, R.R.; Gireesh, T.M.; Hunnur, R.K.; Margankop, S.B. One-pot synthesis of pyrazoline derivatised carbazoles as antitubercular, anticancer agents, their DNA cleavage and antioxidant activities. Eur. J. Med. Chem. 2011, 46, 4366–4373. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.H.; Chen, J.C.; Ling, G.L.; Lin, T.T.; Sun, M.H. Novel synthesis of palladium (II) complexes derived from 3-arylsydnone-4-carbaldehyde N4-phenylthiosemicarbazones and biological activity. J. Pharm. Pharmacol. 2014, 66, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.H.; Yeh, M.Y. Access to the syntheses of sydnonyl-substituted α, β-unsaturated ketones and 1,3-dihydro-indol-2-ones by modified Knoevenagel reaction. Tetrahedron 2003, 59, 4103–4111. [Google Scholar] [CrossRef]
- Biradar, J.S.; Sasidhar, B.S. Solvent-free, microwave assisted Knoevenagel condensation of novel 2,5-disubstituted indole analogues and their biological evaluation. Eur. J. Med. Chem. 2011, 46, 6112–6118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Santos, I.O.; Gaur, P.; Ferreira, V.F.; Garcia, C.R.S.; da Rocha, D.R. Addition of thiols to o-quinone methide: New 2-hydroxy-3-phenylsulfanylmethyl [1,4]naphthoquinones and their activity against the human malaria parasite Plasmodium falciparum (3D7). Eur. J. Med. Chem. 2013, 59, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, M.; Jung, M.; Park, Y.; Kim, M.H.; Park, H.G. Efficient synthetic method of Psammaplin A. Tetrahedron Lett. 2012, 53, 4209–4211. [Google Scholar] [CrossRef]
- Parida, K.M.; Mallick, S.; Sahoo, P.C.; Rana, S.K. A facile method for synthesis of amine-functionalized mesoporous zirconia and its catalytic evaluation in Knoevenagel condensation. Appl. Catal. A Gen. 2010, 381, 226–232. [Google Scholar] [CrossRef]
- Sharma, R.K.; Monga, Y.; Puri, A. Zirconium (IV)-modified silica@magnetic nanocomposites: Fabrication, characterization and application as efficient, selective and reusable nanocatalysts for Friedel-Crafts, Knoevenagel and Pechmann condensation. Catal. Commun. 2013, 35, 110–114. [Google Scholar] [CrossRef]
- Kumari, K.; Raghuvanshi, D.S.; Krishna Nand Singh, K.N. An efficient synthesis of 2H-chromen-3-yl derivatives via CuI/(NH4)2HPO4 catalyzed reaction of O-propargyl salicylaldehydes with active methylene compounds. Tetrahedron 2013, 69, 82–88. [Google Scholar] [CrossRef]
- Kavali, J.R.; Badami, B.V. 1,5-Benzodiazepine derivatives of 3-arylsydnones: Synthesis and antimicrobial activity of 3-aryl-4-[2'-aryl-2'-4',6',7'-tetrahydro-(1'H)-1',5'-benzodiazepine-4'-yl]sydnones. Il Farmaco 2000, 55, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Abd EI-Aal, R.M.; Younis, M. Synthesis and antimicrobial activity of meso-substituted polymethine cyanine dyes. Bioorg. Chem. 2004, 32, 193–210. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, M.-H.; Xu, Y.-Y.; Yang, Y.-S.; Lin, G.-L. A Facile Synthesis and Antimicrobial Activity Evaluation of Sydnonyl-Substituted Thiazolidine Derivatives. Molecules 2015, 20, 6520-6532. https://doi.org/10.3390/molecules20046520
Shih M-H, Xu Y-Y, Yang Y-S, Lin G-L. A Facile Synthesis and Antimicrobial Activity Evaluation of Sydnonyl-Substituted Thiazolidine Derivatives. Molecules. 2015; 20(4):6520-6532. https://doi.org/10.3390/molecules20046520
Chicago/Turabian StyleShih, Mei-Hsiu, Yu-Yuan Xu, Yu-Sheng Yang, and Guan-Ling Lin. 2015. "A Facile Synthesis and Antimicrobial Activity Evaluation of Sydnonyl-Substituted Thiazolidine Derivatives" Molecules 20, no. 4: 6520-6532. https://doi.org/10.3390/molecules20046520
APA StyleShih, M.-H., Xu, Y.-Y., Yang, Y.-S., & Lin, G.-L. (2015). A Facile Synthesis and Antimicrobial Activity Evaluation of Sydnonyl-Substituted Thiazolidine Derivatives. Molecules, 20(4), 6520-6532. https://doi.org/10.3390/molecules20046520