Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid
Abstract
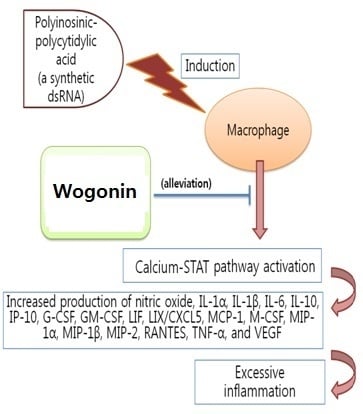
:1. Introduction

2. Results and Discussion
2.1. Effects of Wogonin on Cell Viability
2.2. Effects of Wogonin on NO Production

2.3. Effects of Wogonin on Cytokine Production


2.4. Effects of Wogonin on Intracellular Calcium Release
2.5. Effect of Wogonin on mRNA Expression of STAT1 and STAT3


3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Cell Viability Assay
3.2.2. Quantification of NO Production
3.2.3. Multiplex Bead-Based Cytokine Assay
3.2.4. Intracellular Calcium Assay
3.2.5. RNA Isolation and Real Time RT-PCR Analysis
3.2.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, H.M.; Hong, J.S. Why neurodegenerative diseases are progressive: Uncontrolled inflammation drives disease progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Kim, H.J.; Ramirez, M.; Salameh, S.; Ma, X. The septic shock-associated IL-10-1082 A > G polymorphism mediates allele-specific transcription via poly(ADP-ribose) polymerase 1 in macrophages engulfing apoptotic cells. J. Immunol. 2010, 184, 3718–3724. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.L.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.A.; Morris, I.R.; Berton, M.T. Phosphatidylinositol 3-kinase activation attenuates the TLR2-mediated macrophage proinflammatory cytokine response to francisella tularensis live vaccine strain. J. Immunol. 2010, 185, 7562–7572. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Shiloh, M.U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 2000, 97, 8841–8848. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Ma, A.; Lipsky, P. Cytokines and autoimmunity. Nat. Rev. Immunol. 2002, 2, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ware, C.F. Network communications: Lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 2005, 23, 787–819. [Google Scholar] [CrossRef] [PubMed]
- Sospedra, M.; Martin, R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005, 23, 683–747. [Google Scholar] [CrossRef] [PubMed]
- Ulevitch, R.J.; Tobias, P.S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 1995, 13, 437–457. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Morgan, W.A.; Sanchez-Medina, A.; Corcoran, O. The ethanol extract of scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and bax in human lung cancer cells. Toxicol. Appl. Pharmacol. 2011, 254, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-inflammatory effect of myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid. Molecules 2011, 16, 7132–7142. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Yuk, S.S.; Lim, E.M.; Lee, J.Y.; Lee, Y.J.; Kim, Y.S.; Lee, T.H.; Park, S.K.; Bae, H.; Kim, H.M.; Ko, S.G.; et al. Antiinflammatory effects of epimedium brevicornum water extract on lipopolysaccharide-activated RAW264.7 macrophages. Phytother. Res. 2010, 24, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Miles, K.; Clarke, D.J.; Lu, W.; Sibinska, Z.; Beaumont, P.E.; Davidson, D.J.; Barr, T.A.; Campopiano, D.J.; Gray, M. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J. Immunol. 2009, 183, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.T.; Wang, Q.; Moncada, S.; Singer, M. Hypoxia accelerates nitric oxide-dependent inhibition of mitochondrial complex I in activated macrophages. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R394–R400. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, E.A. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol. 2006, 147, S193–S201. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Zernecke, A.; Libby, P. The multifaceted contributions of leukocyte subsets to atherosclerosis: Lessons from mouse models. Nat. Rev. Immunol. 2008, 8, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Sessler, C.N.; Shepherd, W. New concepts in sepsis. Curr. Opin. Crit. Care 2002, 8, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Karl, I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003, 348, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, L.; Tracey, K.J. The “cytokine profile”: A code for Sepsis. Trends Mol. Med. 2005, 11, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Riedemann, N.C.; Neff, T.A.; Guo, R.F.; Bernacki, K.D.; Laudes, I.J.; Sarma, J.V.; Lambris, J.D.; Ward, P.A. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J. Immunol. 2003, 170, 503–507. [Google Scholar] [CrossRef]
- Dace, D.S.; Khan, A.A.; Stark, J.L.; Kelly, J.; Cross, A.H.; Apte, R.S. Interleukin-10 overexpression promotes fas-ligand-dependent chronic macrophage-mediated demyelinating polyneuropathy. PLoS ONE 2009, 4, e7121. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Jung, S.; Wilhelm, J.; Fink, L.; Buhling, F.; Welte, T.; Bohle, R.M.; Seeger, W.; Lohmeyer, J.; Maus, U.A. The inflammatory versus constitutive trafficking of mononuclear phagocytes into the alveolar space of mice is associated with drastic changes in their gene expression profiles. J. Immunol. 2005, 175, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Fillion, I.; Ouellet, N.; Simard, M.; Bergeron, Y.; Sato, S.; Bergeron, M.G. Role of chemokines and formyl peptides in pneumococcal pneumonia-induced monocyte/macrophage recruitment. J. Immunol. 2001, 166, 7353–7361. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Wing, K. Immunology. Damping by Depletion. Science 2011, 332, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Dayer, J.M.; Choy, E. Therapeutic targets in rheumatoid arthritis: The interleukin-6 receptor. Rheumatology 2010, 49, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wellby, M.L.; Kennedy, J.A.; Pile, K.; True, B.S.; Barreau, P. Serum interleukin-6 and thyroid hormones in rheumatoid arthritis. Metabolism 2001, 50, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Ballara, S.; Taylor, P.C.; Reusch, P.; Marme, D.; Feldmann, M.; Maini, R.N.; Paleolog, E.M. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis Rheum. 2001, 44, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Funk, M.; Karl, D.; Georgopoulos, M.; Benesch, T.; Sacu, S.; Polak, K.; Zlabinger, G.J.; Schmidt-Erfurth, U. Neovascular age-related macular degeneration: Intraocular cytokines and growth factors and the influence of therapy with ranibizumab. Ophthalmology 2009, 116, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Maskrey, B.H.; Megson, I.L.; Whitfield, P.D.; Rossi, A.G. Mechanisms of resolution of inflammation: A focus on cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Wareing, M.D.; Lyon, A.B.; Lu, B.; Gerard, C.; Sarawar, S.R. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza a virus infection in mice. J. Leukoc. Biol. 2004, 76, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, M.J.; Shen, F.; Smith, J.B.; Sharma, A.; Gaffen, S.L. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: Implications for inflammation and neutrophil recruitment. J. Leukoc. Biol. 2004, 76, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Schon, M.P.; Boehncke, W.H. Psoriasis. N. Engl. J. Med. 2005, 352, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.P.; Feng, J.T.; Tang, Y.L.; Zhu, J.Q.; Lin, M.J.; Yu, M.E. LIF upregulates expression of NK-1R in NHBE cells. Mediators Inflamm. 2006, 2006. [Google Scholar] [CrossRef]
- Cuschieri, J.; Maier, R.V. Oxidative stress, lipid rafts, and macrophage reprogramming. Antioxid. Redox Signal 2007, 9, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-dependent protein kinase II Links ER stress with fas and mitochondrial apoptosis pathways. J. Clin. Investig. 2009, 119, 2925–2941. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, L.R.; Brandt, S.L.; Wang, S.; Wang, Z.; Morris, D.L.; Evans-Molina, C.; Mirmira, R.G.; Jancar, S.; Serezani, C.H. Leukotriene B4-mediated sterile inflammation promotes susceptibility to sepsis in a mouse model of type 1 diabetes. Sci. Signal. 2015, 8. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compound (wogonin) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2015, 20, 6888-6900. https://doi.org/10.3390/molecules20046888
Lee JY, Park W. Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules. 2015; 20(4):6888-6900. https://doi.org/10.3390/molecules20046888
Chicago/Turabian StyleLee, Ji Young, and Wansu Park. 2015. "Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid" Molecules 20, no. 4: 6888-6900. https://doi.org/10.3390/molecules20046888
APA StyleLee, J. Y., & Park, W. (2015). Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules, 20(4), 6888-6900. https://doi.org/10.3390/molecules20046888