Chilean Prosopis Mesocarp Flour: Phenolic Profiling and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Prosopis Flour Characterization and Antioxidant Activity

| Sample Origin | % Flour to Pod Ratio | Flour Color | TP(g GAE/100 g FW) | TF(g QE/100 g FW) | % XAD-Retained PEFE | DPPH SC50 (µg PEFE/mL) | FRAP (mMoles TE/g PEFE) | TEAC (μM TE/g PEFE) |
|---|---|---|---|---|---|---|---|---|
| Copiapó valley | ||||||||
| Puquio | 36.7 | Light greyish tan | 2.54 ± 0.12 | n.d. a | 4.26 | 70.51 | 0.50 ± 0.01 | 267.5 |
| Huasco valley | ||||||||
| Alto del Carmen | 15.4 | Light tan | 2.57 ± 0.09 | 0.38 ± 0.07 | 0.19 | 12.07 | 3.45 ± 0.06 | 3206.6 |
| El Tránsito | 3.3 | Light tan | 1.33 ± 0.06 | 0.25 ± 0.01 | 1.63 | 52.85 | 0.65 ± 0.04 | 428.6 |
| Pinte | 15.5 | Light tan | 0.82 ± 0.03 | 0.17 ± 0.01 | 0.91 | 52.97 | 0.63 ± 0.06 | 530.5 |
| Plaza de Pinte | 17.8 | Light tan | 2.11 ± 0.10 | 0.56 ± 0.10 | 0.10 | 23.74 | 1.21 ± 0.08 | Inactive |
| Elqui valley | ||||||||
| Elqui valley | 31.8 | Pale tan | 0.89 ± 0.11 | 0.23 ± 0.03 | 2.08 | >100 | 0.36 ± 0.01 | Inactive |
| Quercetin b | 7.82 ± 0.30 | 10.77 ± 0.16 | 8157.9 |

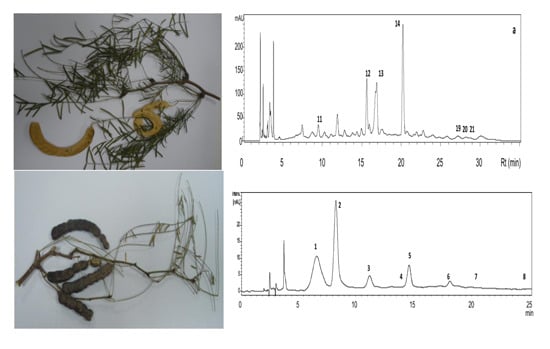
2.2. HPLC-DAD-MS/MS Analysis



| Compound | Rt (min) | UV λmax (nm) | [M]+ (m/z) | MS/MS (m/z) | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 6.2 | 516, 425 sh, 279, 224 | 449 | 287 | Cyanidin 3-hexoside |
| 2 | 8.3 | 516, 425 sh, 279, 224 | 449 | 287 | Cyanidin 3-hexoside |
| 3 | 11.1 | 517, 321 sh, 278, 225 | 463 | 301,286,258 | Peonidin 3-hexoside |
| 4 | 13.1 | - | 479 | 420,317 | Petunidin hexoside |
| 5 | 14.6 | 517, 307sh, 285, 228 | 535 | 287 | Cyanidin malonyl hexoside |
| 6 | 18.2 | 517, 320 sh, 279, 228 | 549 | 505,301 | Peonidin malonyl hexoside |
| 7 | 20.8 | 520, 319 sh, 270, 229 | 463 | 301,286,258 | Peonidin 3-hexoside |
| 8 | 24.1 | - | 493 | 331,315,270 | Malvidin hexoside |
| Compound | Rt (min) | UV λmax (nm) | MW | [M-H] ‾ and Fragment Ions | Tentative Identification |
|---|---|---|---|---|---|
| 9 | 4.8 | - | 464 | 463, 301 | Ellagic acid hexoside |
| 10 | 5.8–6.1 | 289, 228 | 372 | 371, 209, 163 | Hydroxyferulic acid hexoside |
| 11 | 6.8 | 278 | 464 | 463, 301 | Ellagic acid hexoside |
| 12 | 15.4 | 334, 270 | 594 | 593, 473 | Vicenin II/Isomer |
| 13 | 16.7 | 334, 271 | 594 | 593, 473 | Vicenin II/Isomer |
| 14 | 20.0 | 333, 270 | 564 | 563, 503, 473, 443, 383 | Schaftoside/isoschaftoside |
| 15 | 20.1 | 352, 262 | 626 | 625, 300 | Q-dihexoside |
| 16 | 22.5 | 352, 267 sh, 256 | 596 | 595, 463, 301 | Q-hexosidepentoside |
| 17 | 24.4 | 354, 268 sh, 254 | 624 | 623, 315 | Q-methyl ether rhamnoside hexoside |
| 18 | 24.9 | 355, 297sh, 267sh, 254 | 610 | 609, 301 | Q-rutinoside (rhamnoside hexoside) |
| 19 | 26.8–27.3 | - | 432 | 431, 311 | Isovitexin |
| 20 | 28.3 | 352, 265 sh, 254 | 610 | 609, 301 | Q-rhamnoside-hexoside |
| 21 | 28.6 | 349, 267 sh, 253 | 624 | 623, 419, 315 | Q-methyl ether rhamnoside hexoside |
3. Experimental Section
3.1. Chemicals
3.2. Plant Material and Sample Preparation
3.3. Total Phenolic (TP) and Total Flavonoid (TF) Contents
3.4. Antioxidant Activity
3.5. HPLC-DAD-MS Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fagg, C.; Stewart, J. The value of Acacia and Prosopis in arid and semi-arid environments. J. Arid Environ. 1994, 27, 3–25. [Google Scholar] [CrossRef]
- Felger, R.S. Mesquite in Indian Cultures of South-Western North America. In Mesquite: Its Biology in Two Desert Ecosystems, 1st ed.; Simpson, B.B., Ed.; Dowden, Hutchinson and Ross: Stroudsburg, PA, USA, 1977; pp. 150–176. [Google Scholar]
- Schmeda-Hirschmann, G. Plant resources used by the Ayoreo of the Paraguayan Chaco. Econ. Bot. 1994, 48, 252–258. [Google Scholar] [CrossRef]
- Schmeda Hirschmann, G. Etnobotánica Ayoreo. Contribución al estudio de la flora y vegetación del Chaco. XI. Candollea 1998, 53, 1–50. [Google Scholar]
- Pérez, M.J.; Cuello, A.S.; Zampini, I.C.; Ordoñez, R.M.; Alberto, M.R.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Polyphenolic compounds and anthocyanin content of Prosopis nigra and Prosopis alba pods flour and their antioxidant and anti-inflammatory capacity. Food Res. Int. 2014, 64, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Astudillo, L.; Schmeda-Hirschmann, G.; Herrera, J.P.; Cortés, M. Proximate composition and biological activity of Chilean Prosopis species. J. Sci. Food Agric. 2000, 80, 567–573. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Razmilic, I.; Gutierrez, M.I.; Loyola, J.I. Proximate composition and biological activity of food plants gathered by Chilean Amerindians. Econ. Bot. 1999, 53, 177–187. [Google Scholar] [CrossRef]
- Arenas, P. Etnografía y alimentación entre los Toba-Ñachilamolekek y Wichí-Lhukútas del Chaco Central. (Argentina), 1st ed.; ProBiota Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata: Buenos Aires, Argentina, 2003. [Google Scholar]
- Cardozo, M.L.; Ordóñez, R.M.; Zampini, I.C.; Cuello, A.S.; Dibenedetto, G.; Isla, M.I. Evaluation of antioxidant capacity, genotoxicity and polyphenol content of non-conventional food: Prosopis flour. Food Res. Int. 2010, 43, 1505–1510. [Google Scholar] [CrossRef]
- Escobar, B.; Estévez, A.M.; Fuentes, C.; Venegas, D. Use of Algarrobo (Prosopis chilensis (Mol) Stuntz) flour as protein and dietary fiber source in cookies and fried chips manufacture. Arch. Latinoam. Nutr. 2009, 59, 191–198. [Google Scholar]
- Giovannetti, M.A.; Lema, V.S.; Bartoli, C.G.; Capparelli, A. Starch grain characterization of Prosopis chilensis (Mol.) Stuntz and P. flexuosa DC, and the analysis of their archaeological remains in Andean South America. J. Archaeol. Sci. 2008, 35, 2973–2985. [Google Scholar]
- Fuentes, V. Productos Forestales no madereros. INFOR 2013, 16, 1–6. [Google Scholar]
- Soto, D.; Gysling, J. Productos con oportunidades de desarrollo en Chile: Mucílago de algarrobo chileno (Prosopis chilensis). INFOR 2009, 15, 255–276. [Google Scholar]
- Felker, P.A.; Takeoka, G.; Dao, L. Pod mesocarp flour of North and South American species of leguminous tree Prosopis (Mesquite): Composition and food applications. Food Rev. Int. 2013, 29, 49–66. [Google Scholar] [CrossRef]
- Felker, P.; Grados, N.; Cruz, G.; Prokopiuk, D. Economic assessment of production of flour from Prosopis alba and P. pallida pods for human food applications. J. Arid Environ. 2003, 53, 517–528. [Google Scholar] [CrossRef]
- Astudillo, L.; Jürgens, K.; Schmeda-Hirschmann, G.; Griffith, G.A.; Holt, D.J.; Jenkins, P.R. DNA binding alkaloids from Prosopis alba. Planta Med. 1999, 65, 161–162. [Google Scholar]
- Tapia, A.; Feresin, G.E.; Bustos, D.; Astudillo, L.; Theoduloz, C.; Schmeda-Hirschmann, G. Biologically active alkaloids and a free radical scavenger from Prosopis species. J. Ethnopharmacol. 2000, 241–246. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Jakupovic, J. A DNA binding compound from Prosopis tamarugo pods. Bol. Soc. Chil. Quim. 2000, 45, 645–647. [Google Scholar]
- Jain, M.; Jos, E.M.; Arora, D.; Sharma, Y.V.R.K. Effect of proline on Triticum aestivum (wheat) under the drought conditions of salinity. J. Pharm. Res. 2013, 7, 506–509. [Google Scholar] [CrossRef]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, C.A.; Bravo, L.A.; Pinto, M.; Cardemil, L. Physiological and molecular responses of Prosopis chilensis under field and simulation conditions. Phytochemistry 1995, 40, 1375–1382. [Google Scholar] [CrossRef]
- Cattaneo, F.; Sayago, J.E.; Alberto, M.R.; Zampini, I.C.; Ordoñez, R.M.; Chamorro, V.; Pazos, A.; Isla, M.I. Anti-inflammatory and antioxidant activities, functional properties and mutagenicity studies of protein and protein hydrolysate obtained from Prosopis alba seed flour. Food Chem. 2014, 161, 391–399. [Google Scholar] [CrossRef]
- Quispe, C.; Petroll, K.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant effect and characterization of South American Prosopis pods syrup. Food Res. Int. 2014, 56, 174–181. [Google Scholar] [CrossRef]
- Lv, J.; Yu, L.; Lu, Y.; Niu, Y.; Liu, L.; Costa, J.; Yu, L. Phytochemical compositions, and antioxidant properties, and antiproliferative activities of wheat flour. Food Chem. 2012, 135, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mojica, L.; Meyer, A.; Berhow, M.A.; González de Mejía, E. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Kunyanga, C.N.; Imungi, J.K.; Okoth, M.W.; Biesalski, H.K.; Vadivel, V. Total phenolic content, antioxidant and antidiabetic properties of methanolic extract of raw and traditionally processed Kenyan indigenous food ingredients. LWT-Food Sci. Technol. 2012, 45, 269–276. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanins in cereals. J. Chromatogr. A 2004, 1054, 129–141. [Google Scholar]
- Wu, X.; Prior, R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impact on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar]
- Harbaum, B.; Hubbermann, E.M.; Wolff, C.; Herges, R.; Zhu, Z.; Schwarz, K. Identification of flavonoids and hydroxycinnamic acids in Pak Choi varieties (Brassica campestris L. ssp. chinensis var. communis) by HPLC-ESI-MSn and NMR and their quantification by HPLC-DAD. J. Agric. Food Chem. 2007, 55, 8251–8260. [Google Scholar]
- Ferreres, F.; Silva, B.M.; Andrade, P.B.; Seabra, R.M.; Ferreira, M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: Application to seeds of quince (Cydoniaoblonga). Phytochem. Anal. 2003, 14, 352–359. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T.; Full, G.H.; Wong, R.Y.; Harden, L.; Edwards, R.; Berrios, J. Characterization of black bean (Phaseolus vulgaris, L) anthocyanins. J. Agric. Food Chem. 1997, 45, 3395–3400. [Google Scholar] [CrossRef]
- Kamiya, H.; Yanase, E.; Nakatsuka, S. Novel oxidation products of cyanidin 3-O-glucoside with 2,2'-azobis-(2,4-dimethyl) valeronitrile and evaluation of anthocyanin content and its oxidation in black rice. Food Chem. 2014, 155, 221–226. [Google Scholar] [CrossRef] [PubMed]
- De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. LC-MS analysis of anthocyanins from purple corn cob. J. Sci. Food Agric. 2002, 82, 1003–1006. [Google Scholar] [CrossRef]
- Ha, T.J.; Lee, M.H.; Park, C.H.; Pae, S.B.; Shim, K.B.; Ko, J.M.; Shin, S.O.; Baek, I.Y.; Park, K.Y. Identification and characterization of anthocyanins in yard-long beans (Vigna unguiculata ssp. sesquipedalis L.) by high-performance liquid chromatography with diode array detect ion and electrospray ionization/mass spectrometry (HPLC-DAD-ESI/MS) analysis. J. Agric. Food Chem. 2010, 58, 2571–2576. [Google Scholar]
- Takeoka, G.R.; Wong, R.Y.; Dao, L.; Felker, P. Identification of 5,6-dihydro-6-propyl-2H-pyran-2-one as the major volatile constituent in mesquite (Prosopis) flour. Food Chem. 2009, 115, 1025–1027. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.Z.; Chen, M.; Wu, M.J.; Guo, H.L.; Sun, L.X.; Wang, H.; Zhang, S.; Wang, T.; Zhang, L.Y. Protective effect of total flavonoid C-glycosides from Abrus mollis extract on lipopolysaccharide-induced lipotoxicity in mice. Chin. J. Nat. Med. 2014, 12, 461–468. [Google Scholar] [PubMed]
- Chen, M.; Wang, T.; Jiang, Z.Z.; Shan, C.; Wang, H.; Wu, M.J.; Zhang, S.; Zhang, Y.; Zhang, L.Y. Anti-inflammatory and hepatoprotective effects of total flavonoid C-glycosides from Abrus mollis extracts. Chin. J. Nat. Med. 2014, 12, 590–598. [Google Scholar] [PubMed]
- Islam, M.N.; Ishita, I.J.; Jung, H.A.; Choi, J.S. Vicenin 2 isolated from Artemisia capillaris exhibited potent anti-glycation properties. Food Chem. Toxicol. 2014, 69, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Nagaprashantha, L.D.; Vatsyayan, R.; Singhal, J.; Fast, S.; Roby, R.; Awasthi, S.; Singhal, S.S. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem. Pharmacol. 2011, 82, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Structure-dependent membrane interaction of flavonoids associated with their bioactivity. Food Chem. 2010, 120, 1089–1096. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; El Mohsen, M.M.A.; Rice-Evans, C. Cellular uptake and metabolism of flavonoids and their metabolites: Implications for their bioactivity. Arch. Biochem. Biophys. 2004, 423, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aspee, F.; Quispe, C.; Soriano, M.D.P.C.; Fuentes Gonzalez, J.; Hüneke, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and characterization of constituents in Copao fruits (Eulychnia acida Phil., Cactaceae) by HPLC-DAD-MS/MSn. Food Res. Int. 2014, 62, 286–298. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Bórquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and Luma chequén. Food Chem. 2013, 289–299. [Google Scholar] [CrossRef]
- Sample Availability: Samples are not available from authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmeda-Hirschmann, G.; Quispe, C.; Soriano, M.D.P.C.; Theoduloz, C.; Jiménez-Aspée, F.; Pérez, M.J.; Cuello, A.S.; Isla, M.I. Chilean Prosopis Mesocarp Flour: Phenolic Profiling and Antioxidant Activity. Molecules 2015, 20, 7017-7033. https://doi.org/10.3390/molecules20047017
Schmeda-Hirschmann G, Quispe C, Soriano MDPC, Theoduloz C, Jiménez-Aspée F, Pérez MJ, Cuello AS, Isla MI. Chilean Prosopis Mesocarp Flour: Phenolic Profiling and Antioxidant Activity. Molecules. 2015; 20(4):7017-7033. https://doi.org/10.3390/molecules20047017
Chicago/Turabian StyleSchmeda-Hirschmann, Guillermo, Cristina Quispe, Maria Del Pilar C. Soriano, Cristina Theoduloz, Felipe Jiménez-Aspée, Maria Jorgelina Pérez, Ana Soledad Cuello, and Maria Inés Isla. 2015. "Chilean Prosopis Mesocarp Flour: Phenolic Profiling and Antioxidant Activity" Molecules 20, no. 4: 7017-7033. https://doi.org/10.3390/molecules20047017