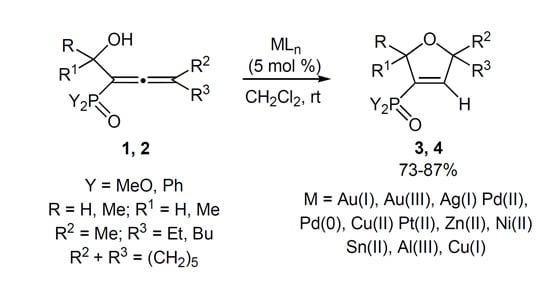
Bifunctionalized Allenes. Part XVI. Synthesis of 3-Phosphoryl-2,5-dihydrofurans by Coinage Metal-Catalyzed Cyclo-isomerization of Phosphorylated α-Hydroxyallenes
Abstract
:1. Introduction
2. Results and Discussion

| Entry | Solvent a | Reaction temperature (°C) | AgNO3 (mol %) | Yield b (%) |
|---|---|---|---|---|
| 1 | ClCH2CH2Cl | −20 | 10 | 41 |
| 2 | ClCH2CH2Cl | 0 | 5 | 55 |
| 3 | ClCH2CH2Cl | rt | 5 | 63 |
| 4 | ClCH2CH2Cl | reflux | 5 | 45 |
| 5 | CHCl3 | rt | 5 | 58 |
| 6 | EtOH | rt | 5 | 46 |
| 7 | MeCN | rt | 5 | 45 |
| 8 | THF | rt | 5 | 40 |
| 9 | toluene | rt | 5 | 28 |
| 10 | acetone | rt | 5 | 51 |
| 11 | acetone/H2O | rt | 5 | 75 |
| 12 | acetone/H2O | rt | 10 | 77 |
| 13 | CH2Cl2 | −20 | 5 | 72 |
| 14 | CH2Cl2 | rt | 5 | 84 c |
| 15 | CH2Cl2 | rt | 10 | 82 |

| Entry | Catalyst | Reaction time a (min) | Yield b (%) | ||
|---|---|---|---|---|---|
| 1a | 2a | 1a | 2a | ||
| 1 | AuCl | 20 | 30 | 97 | 91 |
| 2 | AuCl3 | 30 | 35 | 94 | 89 |
| 3 | AgClO4 | 30 | 55 | 83 c | 85 c |
| 4 | AgNO3 | 50 | 65 | 80 | 80 |
| 5 | PdCl2 | 100 | 115 | 73 | 80 |
| 6 | Pd(PPh3)4 | 105 | 120 | 74 | 77 |
| 7 | CuCl2 | 115 | 110 | 77 | 74 |
| 8 | PtCl2 | 135 | 180 | 66 | 78 |
| 9 | ZnCl2 | 160 | 135 | 50 | 75 |
| 10 | NiCl2 | 225 | 395 | 53 | 36 |
| 11 | SnCl2 | 310 | 255 | 38 | 57 |
| 12 | AlCl3 | 345 | 340 | 34 | 32 |
| 13 | CuCl | 530 | 635 | 27 | 27 |
| 14 | CuBr | 545 | 690 | 29 | 22 |
| 15 | CuI | 600 | 725 | 24 | 23 |
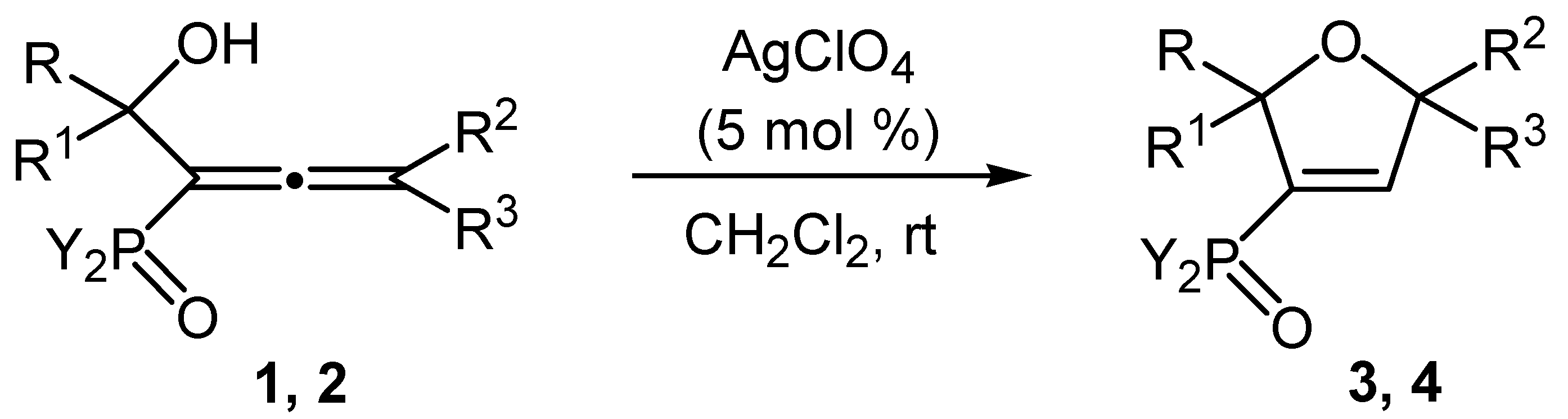
| Entry | Allene | Y | R | R1 | R2 | R3 | Reaction time a (min) | Product, Yield b (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1a | MeO | H | H | Me | Et | 30 | 3a, 83 |
| 2 | 1b | MeO | H | H | Me | Bu | 35 | 3b, 75 |
| 3 | 1c | MeO | H | H | -(CH2)5- | 41 | 3c, 73 | |
| 4 | 1d | MeO | H | Me | Me | Et | 33 | 3d, 77 |
| 5 | 1e | MeO | Me | Me | Me | Bu | 40 | 3e, 74 |
| 6 | 2a | Ph | H | H | Me | Et | 55 | 4a, 85 |
| 7 | 2b | Ph | H | H | Me | Bu | 58 | 4b, 85 |
| 8 | 2c | Ph | H | H | -(CH2)5- | 75 | 4c, 82 | |
| 9 | 2d | Ph | H | Me | Me | Et | 57 | 4d, 84 |
| 10 | 2e | Ph | Me | Me | Me | Bu | 64 | 4e, 82 |
3. Experimental Section
3.1. General Information
3.2. Starting Materials
3.3. General Procedure for the Coinage Metal-catalyzed Cycloisomerization of the 1-Hydroxyalkyl-1,2-dienephosphonates 1
3.4. General Procedure for the Coinage Metal-catalyzed Cycloisomerization of the 2-Diphenylphosphinoyl-2,3-dien-1-ols 2a-c and the 3-Diphenylphosphinoyl-3,4-dien-2-ols 2d,e
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Heaney, H.; Ahn, J.S. Five-membered rings with one heteroatom and fused carbocyclic derivatives. In Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon Press: Oxford, UK, 1996; Volume 2, pp. 297–436. [Google Scholar]
- Eicher, T.; Hauptmann, S. The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Lipshutz, B.H. Five-membered heteroaromatic rings as intermediates in organic synthesis. Chem. Rev. 1986, 86, 795–819. [Google Scholar] [CrossRef]
- Ganguli, M.; Burka, L.T.; Harris, T.M. Structural studies of the mycotoxin verrucosidin. J. Org. Chem. 1984, 49, 3762–3766. [Google Scholar] [CrossRef]
- Franck, B.; Gehrken, H.-P. Citreoviridins from Aspergillus terreus. Angew. Chem. Int. Ed. 1980, 19, 461–462. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Miyake, N.; Kato, K.; Ueno, Y. Peroxyl-radical reaction of retinyl acetate in solution. Biosci. Biotechnol. Biochem. 1992, 56, 1529–1532. [Google Scholar] [CrossRef]
- Boivin, T.L. B. Synthetic routes to tetrahydrofuran, tetrahydropyran, and spiroketal units of polyether antibiotics and a survey of spiroketals of other natural products. Tetrahedron 1987, 43, 3309–3362. [Google Scholar] [CrossRef]
- Koert, U.; Stein, M.; Wagner, H. Bidirectional and convergent routes to oligo(tetrahydrofurans). Chem. Eur. J. 1997, 3, 1170–1180. [Google Scholar] [CrossRef]
- Perron, F.; Albizati, K.F. Chemistry of spiroketals. Chem. Rev. 1989, 89, 1617–1661. [Google Scholar] [CrossRef]
- Erdsack, J.; Krause, N. Synthesis of furanomycin derivatives by Gold-catalyzed cycloisomerization of α-hydroxyallenes. Synthesis 2007, 3741–3750. [Google Scholar] [CrossRef]
- Review on synthesis of dihydrofurans: Kilroy, T.G.; O’Sullivan, T.P.; Guiry, P.J. Synthesis of dihydrofurans substituted in the 2-position. Eur. J. Org. Chem. 2005, 4929–4949. [Google Scholar]
- Buzas, A.; Istrate, F.; Gagosz, F. Gold(I)-catalyzed stereoselective formation of functionalized 2,5-dihydrofurans. Org. Lett. 2006, 8, 1957–1959. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, F.; Song, Z.; Liu, M.; Yan, B. Gold-catalyzed cyclization of (Z)-2-en-4-yn-1-ols: Highly efficient synthesis of fully substituted dihydrofurans and furans. Org. Lett. 2005, 7, 5409–5412. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, R.; Dinesh, C.U.; Nadanan, E.; Hhan, F.A. Palladium-catalyzed reactions of allenes. Chem. Rev. 2000, 100, 3067–3125. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.-I.; Claesson, A. Synthesis of 2,5-dihydrofurans and 5,6-dihydro-2H-pyrans by silver(I)-catalyzed cyclization of allenic alcohols. Synthesis 1979, 743–745. [Google Scholar] [CrossRef]
- Nikam, S.S.; Chu, K.H.; Wang, K.K. The cyclization of trimethylsilyl-substituted α-allenic alcohols to 3-(trimethylsilyl)-2,5-dihydrofurans and their facile autoxidation to 3-(trimethylsilyl)furans or 4-(trimethylsilyl)-2(5H)-furanones. J. Org. Chem. 1986, 51, 745–747. [Google Scholar] [CrossRef]
- Marshall, J.A.; Sehon, C.A. Synthesis of furans and 2,5-dihydrofuransby Ag(I)-catalyzed isomerization of allenones, akynyl allylic alcohols, and allenyl carbinols. J. Org. Chem. 1995, 60, 5966–5968. [Google Scholar] [CrossRef]
- Marshall, J.A.; Yu, R.H.; Perkins, J.F. Diastereo- and enantioselective synthesis of allenylcarbinols through SE2’ addition of transient nonracemic propargylic stannanes to aldehydes. J. Org. Chem. 1995, 60, 5550–5555. [Google Scholar] [CrossRef]
- Chilot, J.-J.; Doutheau, A.; Gore, J. Heterocyclisation de diols βγ’-alleniques. Tetrahedron Lett. 1982, 23, 4693–4696. [Google Scholar] [CrossRef]
- Gelin, R.; Gelin, S.; Albrand, M. Oxymercuration-demercuration d’alcools α-alleniques. Bull. Soc. Chim. Fr. 1972, 1946–1949. [Google Scholar]
- Uemura, K.; Shiraishi, D.; Noziri, M.; Inoue, Y. Preparation of cyclic carbonates from alkadienols, CO2, and aryl or vinylic halides catalyzed by a palladium complex. Bull. Chem. Soc. Jpn. 1999, 72, 1063–1069. [Google Scholar] [CrossRef]
- Kang, S.-K.; Baik, T.-G.; Kulak, A.N. Palladium(0)-catalyzed coupling cyclization of functionalized allenes with hypervalent iodonium salts. Synlett 1999, 324–326. [Google Scholar] [CrossRef]
- Kang, S.-K.; Yamaguchi, T.; Pyun, S.-J.; Lee, Y.-T.; Baik, T.-G. Palladium-catalyzed arylation of α-allenic alcohols with hypervalent iodonium salts: Synthesis of epoxides and diol cyclic carbonates. Tetrahedron Lett. 1998, 39, 2127–2130. [Google Scholar] [CrossRef]
- Ma, S.; Gao, W. Efficient synthesis of 4-(2-alkenyl)-2,5-dihydrofurans via PdCl2-catalyzed coupling-cyclization reaction of 2,3-allenols with allylic halides. Tetrahedron Lett. 2000, 41, 8933–8936. [Google Scholar] [CrossRef]
- Ma, S.; Gao, W. Efficient Synthesis of 4-(2'-alkenyl)-2,5-dihydrofurans and 5,6-dihydro-2H-pyrans via the Pd-catalyzed cyclizative coupling reaction of 2,3- or 3,4-allenols with allylic halides. J. Org. Chem. 2002, 67, 6104–6112. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, E.; Kaneko, T.; Zhang, S.-W.; Onitsuka, K.; Takahashi, S. Ruthenium-catalyzed cycliccarbonylation of allenyl alcohols. Selective synthesis of γ- and δ-lactones. Org. Lett. 2000, 2, 441–443. [Google Scholar]
- Trost, B.M.; Pinkerton, A.B. A Ruthenium-catalyzed alkylative cycloetherification. J. Am. Chem. Soc. 1999, 121, 10842–10843. [Google Scholar] [CrossRef]
- Hoffmann-Röder, A.; Krause, N. The golden gate to catalysis. Org. Biomol. Chem. 2005, 3, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Widenhoefer, R.A.; Han, X. Gold-catalyzed hydroamination of C-C multiple bonds. Eur. J. Org. Chem. 2006, 4555–4563. [Google Scholar] [CrossRef]
- Hashmi, A.S. K.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Jimenez-Nunez, E.; Echavarren, A.M. Molecular diversity through gold catalysis with alkynes. Chem. Commun. 2007, 333–343. [Google Scholar] [CrossRef]
- Gorin, D.J.; Toste, F.D. Relativistic effects in homogeneous Gold catalysis. Nature 2007, 446, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Bongers, N.; Krause, N. Golden opportunities in stereoselective catalysis. Angew. Chem. Int. Ed. 2008, 47, 2178–2181. [Google Scholar] [CrossRef]
- Hoffman-Roder, A.; Krause, N. Gold(III) chloride catalyzed cyclization of α-hydroxyallenes to 2,5-dihydrofurans. Org. Lett. 2001, 3, 2537–2538. [Google Scholar] [CrossRef] [PubMed]
- Krause, N.; Hoffman-Roder, A.; Canisius, J. From amino acids to dihydrofurans: Functionalized allenes in modern organic synthesis. Synthesis 2002, 1759–1774. [Google Scholar] [CrossRef]
- Deutsch, C.; Gockel, B.; Hoffmann-Röder, A.; Krause, N. Golden opportunities in stereoselective catalysis: Optimization of chirality transfer and catalyst efficiency in the Gold-catalyzed cycloisomerization of α-hydroxyallenes to 2,5-dihydrofurans. Synlett 2007, 1790–1794. [Google Scholar] [CrossRef]
- Gockel, B.; Krause, N. Golden times for allenes: Gold-catalyzed cycloisomerization of α-hydroxyallenes to dihydropyrans. Org. Lett. 2006, 8, 4485–4488. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Krause, N. Gold catalysis in organic synthesis: Efficient cycloisomerization of α-aminoallenes to 3-pyrrolines. Org. Lett. 2004, 6, 4121–4123. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Krause, N. Gold-Catalyzed cycloisomerization of α-aminoallenes to 3-pyrrolines—optimization and mechanistic studies. Eur. J. Org. Chem. 2006, 4634–4641. [Google Scholar] [CrossRef]
- Morita, N.; Krause, N. The First Gold-catalyzed C-S bond formation: Cycloisomerization of α-thioallenes to 2,5-dihydrothiophenes. Angew. Chem. Int. Ed. 2006, 45, 1897–1899. [Google Scholar] [CrossRef]
- Angelov, C.M. Five-membered heterocyclization of phosphorus-containing allenes by their reaction with electrophiles—possibilities and restrictions. Phosphorus Sulfur 1983, 15, 177–193. [Google Scholar] [CrossRef]
- Khusainova, N.G.; Pudovik, A.N. Phosphorylated allenes. Methods of synthesis and properties. Russ. Chem. Rev. 1987, 56, 564–578. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Brel, V.K. Phosphorylated allenes: structure and interaction with electrophilic reagents. Russ. Chem. Rev. 1997, 66, 205–224. [Google Scholar] [CrossRef]
- Ma, S. Electrophilic addition and cyclization reactions of allenes. Acc. Chem. Res. 2009, 42, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Brel, V.K. Phosphonoallenes for building organophosphorus derivatives. Heteroatom Chem. 2006, 17, 547–556. [Google Scholar] [CrossRef]
- Brel, V.K. Synthesis and intramolecular cyclization of diethylphosphono-substituted allenic glycols. Synthesis 2001, 1539–1545. [Google Scholar] [CrossRef]
- Brel, V.K. Synthesis and cyclization of diethylphosphono-substituted α-allenic alcohols to 4-(diethylphosphono)-2,5-dihydrofurans. Synthesis 1999, 463–466. [Google Scholar] [CrossRef]
- Brel, V.K.; Abramkin, E.V. Cyclization of allenyl phosphonates to 3-chloro-4-(diethylphosphono)-2,5-dihydrofurans induced by CuCl2. Mendeleev Commun. 2002, 12, 64–66. [Google Scholar] [CrossRef]
- Brel, V.K.; Belsky, V.K.; Stash, A.I.; Zavodnik, V.E.; Stang, P.J. Synthesis and molecular structure of new unsaturated analogues of nucleotides containing six-membered rings. Eur. J. Org. Chem. 2005, 512–521. [Google Scholar] [CrossRef]
- Christov, V.C.; Ivanov, I.K. Alkatrienyl sulfoxides and sulfones. Part VI. Cheletropic addition of sulfur dioxide to 1- and 3-vinylallenyl sulfoxides and sulfones. Heterocycles 2004, 63, 2203–2206. [Google Scholar] [CrossRef]
- Christov, V.C.; Ivanov, I.K.; Ismailov, I.E. Bifunctionalized allenes. Part X. An electrophilic cyclization protocol for convenient highly regioselective synthesis of 3-sulfonyl-furan-2(5H)-ones from 2-sulfonyl-allenoates. Heterocycles 2013, 87, 1903–1916. [Google Scholar] [CrossRef]
- Ivanov, I.K.; Parushev, I.D.; Christov, V.C. Bifunctionalized allenes. Part XII. Electrophilic cyclization and addition reactions of 4-sulfinylated or 4-sulfonylated allenoates. Phosphorus Sulfur 2014, 189, 1503–1513. [Google Scholar] [CrossRef]
- Ivanov, I.K.; Parushev, I.D.; Christov, V.C. Bifunctionalized allenes. Part XI. Competitive electrophilic cyclization and addition reactions of 4-phosphorylated allenecarboxylates. Heteroatom Chem. 2014, 25, 60–71. [Google Scholar] [CrossRef]
- Ismailov, I.E.; Ivanov, I.K.; Christov, V.C. Bifunctionalized allenes. Part XIII. A convenient and efficient method for regioselective synthesis of phosphorylated α-hydroxyallenes with protected and unprotected hydroxy group. Molecules 2014, 19, 6309–6329. [Google Scholar] [CrossRef] [PubMed]
- Ismailov, I.E.; Ivanov, I.K.; Christov, V.C. Bifunctionalized allenes. Part XV. Synthesis of 2,5-dihydro-1,2-oxaphospholes by electrophilic cyclization reaction of phosphorylated α-hydroxyallenes. Molecules 2014, 19, 11056–11076. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1, 2, 3 and 4 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christov, V.C.; Ismailov, I.E.; Ivanov, I.K. Bifunctionalized Allenes. Part XVI. Synthesis of 3-Phosphoryl-2,5-dihydrofurans by Coinage Metal-Catalyzed Cyclo-isomerization of Phosphorylated α-Hydroxyallenes. Molecules 2015, 20, 7263-7275. https://doi.org/10.3390/molecules20047263
Christov VC, Ismailov IE, Ivanov IK. Bifunctionalized Allenes. Part XVI. Synthesis of 3-Phosphoryl-2,5-dihydrofurans by Coinage Metal-Catalyzed Cyclo-isomerization of Phosphorylated α-Hydroxyallenes. Molecules. 2015; 20(4):7263-7275. https://doi.org/10.3390/molecules20047263
Chicago/Turabian StyleChristov, Valerij Ch., Ismail E. Ismailov, and Ivaylo K. Ivanov. 2015. "Bifunctionalized Allenes. Part XVI. Synthesis of 3-Phosphoryl-2,5-dihydrofurans by Coinage Metal-Catalyzed Cyclo-isomerization of Phosphorylated α-Hydroxyallenes" Molecules 20, no. 4: 7263-7275. https://doi.org/10.3390/molecules20047263