BMP Signaling Regulates Bone Morphogenesis in Zebrafish through Promoting Osteoblast Function as Assessed by Their Nitric Oxide Production
Abstract
:1. Introduction
2. Results
2.1. Inhibition of BMP Signaling Starting at 2 or 3 dpf Stages Affects Bone Mineralization



2.2. Type I BMP Receptors Are Required for Osteoblast Formation and Function


2.3. Expression of a Dominant-Negative BMP Receptor Affects Bone Formation
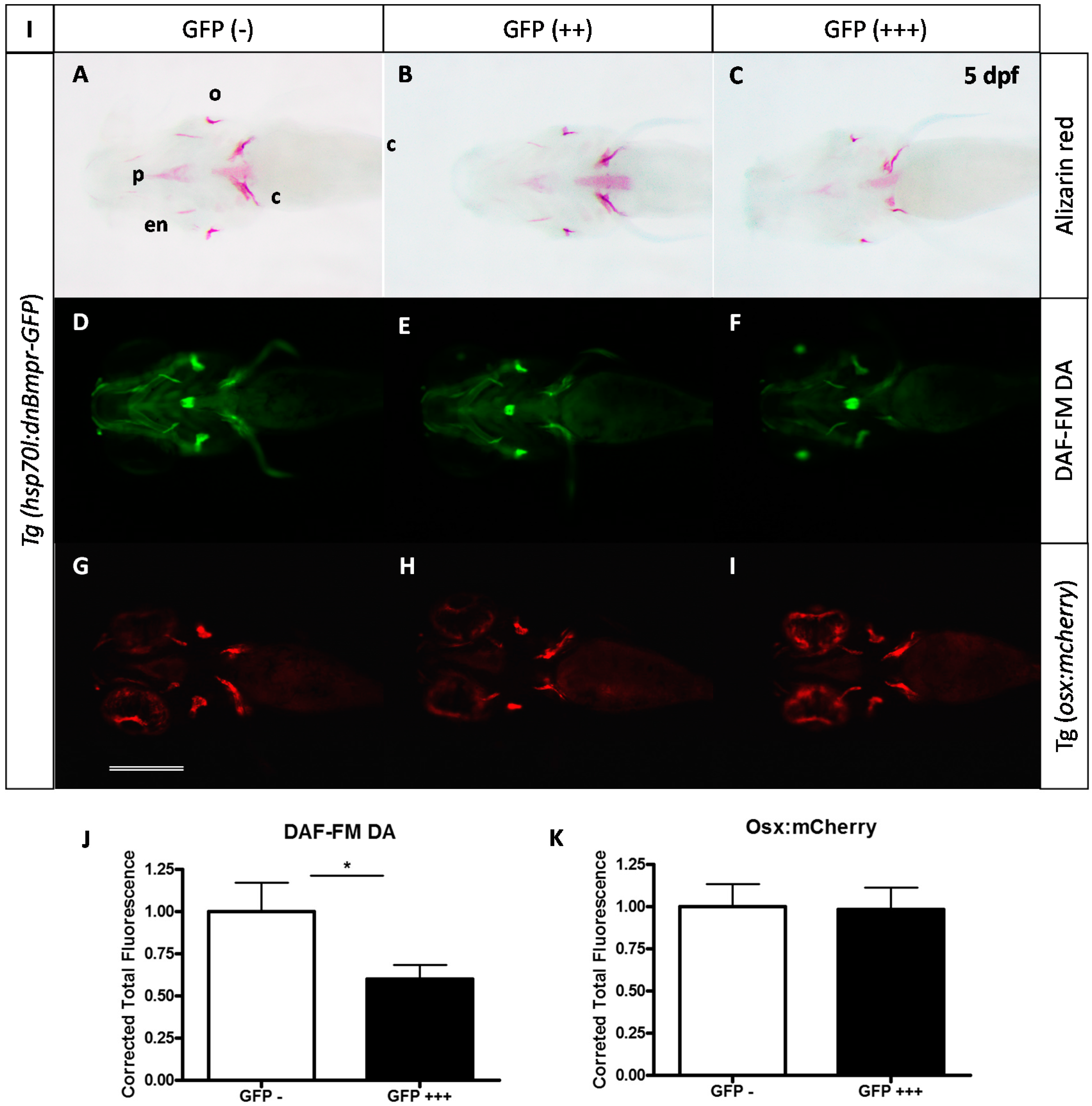
3. Discussion
4. Experimental Section
4.1. Fish and Embryo Maintenance
4.2. Alcian Blue and Alizarin Red Staining
4.3. Inhibition of Bmp Signaling
4.4. Living Nitric Oxide Labeling
4.5. Whole-Mount in Situ Hybridization (WISH)
4.6. Image Aquisition
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schilling, T.F.; Kimmel, C.B. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 1994, 120, 483–494. [Google Scholar] [PubMed]
- Hammond, C.L.; Schulte-Merker, S. Two populations of endochondral osteoblasts with differential sensitivity to hedgehog signalling. Development 2009, 136, 3991–4000. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.V.; Lam, E.Y.; Crosier, P.; Crosier, K. A hierarchy of runx transcription factors modulate the onset of chondrogenesis in craniofacial endochondral bones in zebrafish. Dev. Dyn. 2006, 235, 3166–3176. [Google Scholar] [CrossRef] [PubMed]
- Spoorendonk, K.M.; Peterson-Maduro, J.; Renn, J.; Trowe, T.; Kranenbarg, S.; Winkler, C.; Schulte-Merker, S. Retinoic acid and cyp26b1 are critical regulators of osteogenesis in the axial skeleton. Development 2008, 135, 3765–3774. [Google Scholar] [CrossRef] [PubMed]
- Gavaia, P.J.; Simes, D.C.; Ortiz-Delgado, J.B.; Viegas, C.S.; Pinto, J.P.; Kelsh, R.N.; Sarasquete, M.C.; Cancela, M.L. Osteocalcin and matrix gla protein in zebrafish (Danio rerio) and senegal sole (Solea senegalensis): Comparative gene and protein expression during larval development through adulthood. Gene Expr. Patterns 2006, 6, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Felber, K.; Elks, P.; Croucher, P.; Roehl, H.H. Tracking gene expression during zebrafish osteoblast differentiation. Dev. Dyn. 2009, 238, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Apschner, A.; Schulte-Merker, S.; Witten, P.E. Not all bones are created equal—Using zebrafish and other teleost species in osteogenesis research. Methods Cell Biol. 2011, 105, 239–255. [Google Scholar] [PubMed]
- Vanoevelen, J.; Janssens, A.; Huitema, L.F.; Hammond, C.L.; Metz, J.R.; Flik, G.; Voets, T.; Schulte-Merker, S. Trpv5/6 is vital for epithelial calcium uptake and bone formation. FASEB J. 2011, 25, 3197–3207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huitema, L.F.; Apschner, A.; Logister, I.; Spoorendonk, K.M.; Bussmann, J.; Hammond, C.L.; Schulte-Merker, S. Entpd5 is essential for skeletal mineralization and regulates phosphate homeostasis in zebrafish. Proc. Natl. Acad. Sci. USA 2012, 109, 21372–21377. [Google Scholar] [CrossRef] [PubMed]
- Apschner, A.; Huitema, L.F.; Ponsioen, B.; Peterson-Maduro, J.; Schulte-Merker, S. Zebrafish enpp1 mutants exhibit pathological mineralization, mimicking features of generalized arterial calcification of infancy (GACI) and pseudoxanthoma elasticum (PXE). Dis. Models Mech. 2014, 7, 811–822. [Google Scholar] [CrossRef]
- Poulain, M.; Furthauer, M.; Thisse, B.; Thisse, C.; Lepage, T. Zebrafish endoderm formation is regulated by combinatorial nodal, fgf and bmp signalling. Development 2006, 133, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Luukko, K.; Kettunen, P. Bmp signalling in craniofacial development. Int. J. Dev. Biol. 2006, 50, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Gomis, R.R. The logic of TGFβ signaling. FEBS Lett. 2006, 580, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Dudas, M.; Sridurongrit, S.; Nagy, A.; Okazaki, K.; Kaartinen, V. Craniofacial defects in mice lacking BMP type i receptor Alk2 in neural crest cells. Mech. Dev. 2004, 121, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.O.; Chung, I.H.; Xu, X.; Oka, S.; Zhao, H.; Cho, E.S.; Deng, C.; Chai, Y. Smad4 is required to regulate the fate of cranial neural crest cells. Dev. Biol. 2007, 312, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Snider, P.; Firulli, A.B.; Conway, S.J. Trigenic neural crest-restricted smad7 over-expression results in congenital craniofacial and cardiovascular defects. Dev. Biol. 2010, 344, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, Y.J.; Kim, H.J.; Park, H.D.; Kang, A.R.; Kyung, H.M.; Sung, J.H.; Wozney, J.M.; Kim, H.J.; Ryoo, H.M. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-β1 opposes the Bmp-2-induced osteoblast differentiation by suppression of Dlx5 expression. J. Biol. Chem. 2003, 278, 34387–34394. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kwon, T.G.; Park, H.S.; Wozney, J.M.; Ryoo, H.M. Bmp-2-induced osterix expression is mediated by dlx5 but is independent of runx2. Biochem. Biophys. Res. Commun. 2003, 309, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Keupp, K.; Semler, O.; Wang, W.; Li, Y.; Thiele, H.; Yigit, G.; Pohl, E.; Becker, J.; Frommolt, P.; et al. Attenuated bmp1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am. J. Hum. Genet. 2012, 90, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Holzschuh, J.; Wada, N.; Wada, C.; Schaffer, A.; Javidan, Y.; Tallafuss, A.; Bally-Cuif, L.; Schilling, T.F. Requirements for endoderm and bmp signaling in sensory neurogenesis in zebrafish. Development 2005, 132, 3731–3742. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Barbera, J.P.; Toresson, H.; Da Rocha, S.; Krauss, S. Cloning and expression of three members of the zebrafish bmp family: Bmp2a, bmp2b and bmp4. Gene 1997, 198, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Tucker, A.S. Fgf and bmp signals repress the expression of bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint. Dev. Biol. 2004, 266, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Zuniga, E.; Blitz, I.L.; Wada, N.; Le Pabic, P.; Javidan, Y.; Zhang, T.; Cho, K.W.; Crump, J.G.; Schilling, T.F. Combinatorial roles for bmps and endothelin 1 in patterning the dorsal-ventral axis of the craniofacial skeleton. Development 2011, 138, 5135–5146. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Daleo, M.A.; Murphy, C.K.; Yu, P.B.; Ho, J.N.; Hu, J.; Peterson, R.T.; Hatzopoulos, A.K.; Hong, C.C. Dorsomorphin, a selective small molecule inhibitor of bmp signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS ONE 2008, 3, e2904. [Google Scholar] [CrossRef] [PubMed]
- Boergermann, J.H.; Kopf, J.; Yu, P.B.; Knaus, P. Dorsomorphin and ldn-193189 inhibit bmp-mediated smad, p38 and akt signalling in c2c12 cells. Int. J. Biochem. Cell Biol. 2010, 42, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.B.; Deng, D.Y.; Lai, C.S.; Hong, C.C.; Cuny, G.D.; Bouxsein, M.L.; Hong, D.W.; McManus, P.M.; Katagiri, T.; Sachidanandan, C.; et al. Bmp type i receptor inhibition reduces heterotopic [corrected] ossification. Nat. Med. 2008, 14, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Ho, J.N.; Lewis, J.A.; Karim, K.A.; Daniels, R.N.; Gentry, P.R.; Hopkins, C.R.; Lindsley, C.W.; Hong, C.C. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective vegf and bmp inhibitors. ACS Chem. Biol. 2010, 5, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Sanvitale, C.E.; Kerr, G.; Chaikuad, A.; Ramel, M.C.; Mohedas, A.H.; Reichert, S.; Wang, Y.; Triffitt, J.T.; Cuny, G.D.; Yu, P.B.; et al. A new class of small molecule inhibitor of bmp signaling. PLoS ONE 2013, 8, e62721. [Google Scholar] [CrossRef] [PubMed]
- Renn, J.; Pruvot, B.; Muller, M. Detection of nitric oxide by diaminofluorescein visualizes the skeleton in living zebrafish. J. Appl. Ichthyol. 2014, 30, 701–706. [Google Scholar] [CrossRef]
- Renn, J.; Winkler, C. Osterix-mcherry transgenic medaka for in vivo imaging of bone formation. Dev. Dyn. 2009, 238, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.F.; Concordet, J.P.; Ingham, P.W. Regulation of left-right asymmetries in the zebrafish by shh and bmp4. Dev. Biol. 1999, 210, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Cao, X. Bmp signaling in skeletal development. Biochem. Biophys. Res. Commun. 2005, 328, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Claudio, M.; Wang, J.; Bai, Y.; Klysik, E.; Selever, J.; Martin, J.F. Bmp signaling regulates a dose-dependent transcriptional program to control facial skeletal development. Development 2012, 139, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Crump, J.G. Bmps and id2a act upstream of twist1 to restrict ectomesenchyme potential of the cranial neural crest. PLoS Genet. 2012, 8, e1002710. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, E.; Rippen, M.; Alexander, C.; Schilling, T.F.; Crump, J.G. Gremlin 2 regulates distinct roles of bmp and endothelin 1 signaling in dorsoventral patterning of the facial skeleton. Development 2011, 138, 5147–5156. [Google Scholar] [CrossRef] [PubMed]
- Dalcq, J.; Pasque, V.; Ghaye, A.; Larbuisson, A.; Motte, P.; Martial, J.A.; Muller, M. Runx3, egr1 and sox9b form a regulatory cascade required to modulate bmp-signaling during cranial cartilage development in zebrafish. PLoS ONE 2012, 7, e50140. [Google Scholar] [CrossRef] [PubMed]
- Pyati, U.J.; Webb, A.E.; Kimelman, D. Transgenic zebrafish reveal stage-specific roles for bmp signaling in ventral and posterior mesoderm development. Development 2005, 132, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.E.; Sheehan-Rooney, K.; Dixon, M.J.; Eberhart, J.K. Examination of a palatogenic gene program in zebrafish. Dev. Dyn. 2011, 240, 2204–2220. [Google Scholar] [CrossRef] [PubMed]
- Cheah, F.S.; Winkler, C.; Jabs, E.W.; Chong, S.S. TGFβ3 regulation of chondrogenesis and osteogenesis in zebrafish is mediated through formation and survival of a subpopulation of the cranial neural crest. Mech. Dev. 2010, 127, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.M.; Ralston, S.H. Nitric oxide and bone. J. Bone Miner. Res. 1996, 11, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, R.J.; Ralston, S.H. Nitric oxide and bone. Immunology 2001, 103, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, H.; Miura, M.; Tsuchida, S.; Hata, I.; Hata, K.; Yamamoto, K.; Ishii, Y.; Muramatsu, I.; Sudo, M. Effect of nitric oxide synthase inhibitors on bone metabolism in growing rats. Am. J. Physiol. 1996, 270, E840–E845. [Google Scholar] [PubMed]
- Turner, C.H.; Owan, I.; Jacob, D.S.; McClintock, R.; Peacock, M. Effects of nitric oxide synthase inhibitors on bone formation in rats. Bone 1997, 21, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.; Buttery, L.; O’Shaughnessy, M.; Afzal, F.; Fernandez de Marticorena, I.; Hukkanen, M.; Huang, P.; MacIntyre, I.; Polak, J. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am. J. Pathol. 2001, 158, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Armour, K.E.; Armour, K.J.; Gallagher, M.E.; Godecke, A.; Helfrich, M.H.; Reid, D.M.; Ralston, S.H. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology 2001, 142, 760–766. [Google Scholar] [PubMed]
- Ralston, S.H.; Grabowski, P.S. Mechanisms of cytokine induced bone resorption: Role of nitric oxide, cyclic guanosine monophosphate, and prostaglandins. Bone 1996, 19, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Saura, M.; Tarin, C.; Zaragoza, C. Recent insights into the implication of nitric oxide in osteoblast differentiation and proliferation during bone development. Sci. World J. 2010, 10, 624–632. [Google Scholar] [CrossRef]
- Bacabac, R.G.; Smit, T.H.; Mullender, M.G.; Dijcks, S.J.; Van Loon, J.J.; Klein-Nulend, J. Nitric oxide production by bone cells is fluid shear stress rate dependent. Biochem. Biophys. Res. Commun. 2004, 315, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.D.; Soejima, K.; Klein-Nulend, J.; Burger, E.H. The production of nitric oxide and prostaglandin E2 by primary bone cells is shear stress dependent. J. Biomech. 2001, 34, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, A.A.; Rawlinson, S.C.; Suswillo, R.F.; Bourrin, S.; Zaman, G.; Lanyon, L.E. Mechanical strain-induced no production by bone cells: A possible role in adaptive bone (re)modeling? FASEB J. 1995, 9, 1614–1622. [Google Scholar] [PubMed]
- Rangaswami, H.; Schwappacher, R.; Marathe, N.; Zhuang, S.; Casteel, D.E.; Haas, B.; Chen, Y.; Pfeifer, A.; Kato, H.; Shattil, S.; et al. Cyclic gmp and protein kinase g control a src-containing mechanosome in osteoblasts. Sci. Signal. 2010, 3, ra91. [Google Scholar] [PubMed]
- Rangaswami, H.; Schwappacher, R.; Tran, T.; Chan, G.C.; Zhuang, S.; Boss, G.R.; Pilz, R.B. Protein kinase g and focal adhesion kinase converge on Src/Akt/β-catenin signaling module in osteoblast mechanotransduction. J. Biol. Chem. 2012, 287, 21509–21519. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Keller, K.C.; Martinez, I.K.; Geransar, R.M.; zur Nieden, K.O.; Nishikawa, S.G.; Rancourt, D.E.; zur Nieden, N.I. No-β-catenin crosstalk modulates primitive streak formation prior to embryonic stem cell osteogenic differentiation. J. Cell Sci. 2012, 125, 5564–5577. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.B.; Kimmel, C.B. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 2007, 82, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lepiller, S.; Laurens, V.; Bouchot, A.; Herbomel, P.; Solary, E.; Chluba, J. Imaging of nitric oxide in a living vertebrate using a diamino-fluorescein probe. Free Radic Biol. Med. 2007, 43, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, G.; Gerster, T. Multicolor whole-mount in situ hybridization. Methods Mol. Biol. 2000, 137, 139–148. [Google Scholar] [PubMed]
- Yan, Y.L.; Miller, C.T.; Nissen, R.M.; Singer, A.; Liu, D.; Kirn, A.; Draper, B.; Willoughby, J.; Morcos, P.A.; Amsterdam, A.; et al. A zebrafish sox9 gene required for cartilage morphogenesis. Development 2002, 129, 5065–5079. [Google Scholar] [PubMed]
- ImageJ: Image Processing and Analysis in Java. Natioanl Institutes of Health: Bethesda, MD, USA. Available online: http://imagej.nih.gov/ (accessed on 20 April 2015).
- Sample Availability: All compounds are commercially available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Windhausen, T.; Squifflet, S.; Renn, J.; Muller, M. BMP Signaling Regulates Bone Morphogenesis in Zebrafish through Promoting Osteoblast Function as Assessed by Their Nitric Oxide Production. Molecules 2015, 20, 7586-7601. https://doi.org/10.3390/molecules20057586
Windhausen T, Squifflet S, Renn J, Muller M. BMP Signaling Regulates Bone Morphogenesis in Zebrafish through Promoting Osteoblast Function as Assessed by Their Nitric Oxide Production. Molecules. 2015; 20(5):7586-7601. https://doi.org/10.3390/molecules20057586
Chicago/Turabian StyleWindhausen, Thomas, Steeve Squifflet, Jörg Renn, and Marc Muller. 2015. "BMP Signaling Regulates Bone Morphogenesis in Zebrafish through Promoting Osteoblast Function as Assessed by Their Nitric Oxide Production" Molecules 20, no. 5: 7586-7601. https://doi.org/10.3390/molecules20057586






