Synthesis, Antifungal Activity and Structure-Activity Relationships of Novel 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid Amides
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis of Compounds




2.2. In Vitro Antifungal Activity
| No. | Inhibition Rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| C.O. | R.S. | P.I. | P.A. | F.S. | B.B. | B.C. | |
| 3a | 35.29 | 37.32 | 26.77 | 26.15 | 24.00 | 41.48 | 33.90 |
| 6a | 29.41 | 53.56 | 22.73 | 37.67 | 24.31 | 67.52 | 37.07 |
| 6b | 83.66 | 44.73 | 26.77 | 61.38 | 17.24 | 50.86 | 69.75 |
| 6c | 56.21 | 31.91 | 36.87 | 48.85 | 22.47 | 32.11 | 48.05 |
| 6d | 48.37 | 60.11 | 8.59 | 34.51 | 25.24 | 88.92 | 49.27 |
| 6e | 69.28 | 39.60 | 28.79 | 77.24 | 29.85 | 61.65 | 63.90 |
| 6f | 53.59 | 38.46 | 29.80 | 43.90 | 27.08 | 45.17 | 51.70 |
| 9a | 79.74 | 42.74 | 51.52 | 55.28 | 22.47 | 53.13 | 55.12 |
| 9b | 84.97 | 54.13 | 53.03 | 73.98 | 40.31 | 69.89 | 97.56 |
| 9c | 92.81 | 92.59 | 56.06 | 90.79 | 39.28 | 83.24 | 69.27 |
| 9d | 54.90 | 35.62 | 30.30 | 45.26 | 26.77 | 48.87 | 37.56 |
| 9e | 56.21 | 47.29 | 51.01 | 42.82 | 26.16 | 70.46 | 79.02 |
| 9f | 62.75 | 95.16 | 35.86 | 77.51 | 26.16 | 66.20 | 84.88 |
| 9g | 48.37 | 70.66 | 47.98 | 48.78 | 24.00 | 60.8 | 76.58 |
| 9h | 25.49 | 51.28 | 60.10 | 27.10 | 43.39 | 33.81 | 37.80 |
| 9i | 83.63 | 45.58 | 40.91 | 81.30 | 22.47 | 61.37 | 78.78 |
| 9j | 74.51 | 56.41 | 51.52 | 53.66 | 26.16 | 79.83 | 81.46 |
| 9k | 78.43 | 82.62 | 72.73 | 62.33 | 35.39 | 51.71 | 68.05 |
| 9l | 52.29 | 88.89 | 56.06 | 46.25 | 41.54 | 35.52 | 49.75 |
| 9m | 94.12 | 86.32 | 51.52 | 90.24 | 38.47 | 84.38 | 86.10 |
| 9n | 84.97 | 76.07 | 42.93 | 65.67 | 12.01 | 68.18 | 55.36 |
| Boscalid | 83.61 | 91.74 | 36.36 | 85.64 | 31.08 | 79.55 | 83.66 |
| No. | C.O. | R.S. | P.I. | P.A. | F.S. | B.B. | B.C. |
|---|---|---|---|---|---|---|---|
| 9b | 33.99 | 55.83 | 80.94 | 35.35 | 96.21 | 29.13 | 24.87 |
| 9c | 21.03 | 26.92 | 86.85 | 31.65 | 83.52 | 28.71 | 32.80 |
| 9f | 26.71 | 16.55 | 94.24 | 30.84 | 86.57 | 46.29 | 20.72 |
| 9k | 25.94 | 21.92 | 39.22 | 26.12 | 58.17 | 49.22 | 41.35 |
| 9m | 5.50 | 14.40 | 75.54 | 21.04 | 79.42 | 28.29 | 30.69 |
| Boscalid | 5.86 | 15.48 | 79.80 | 15.58 | 86.28 | 33.39 | 19.69 |
2.3. QSAR Analyses
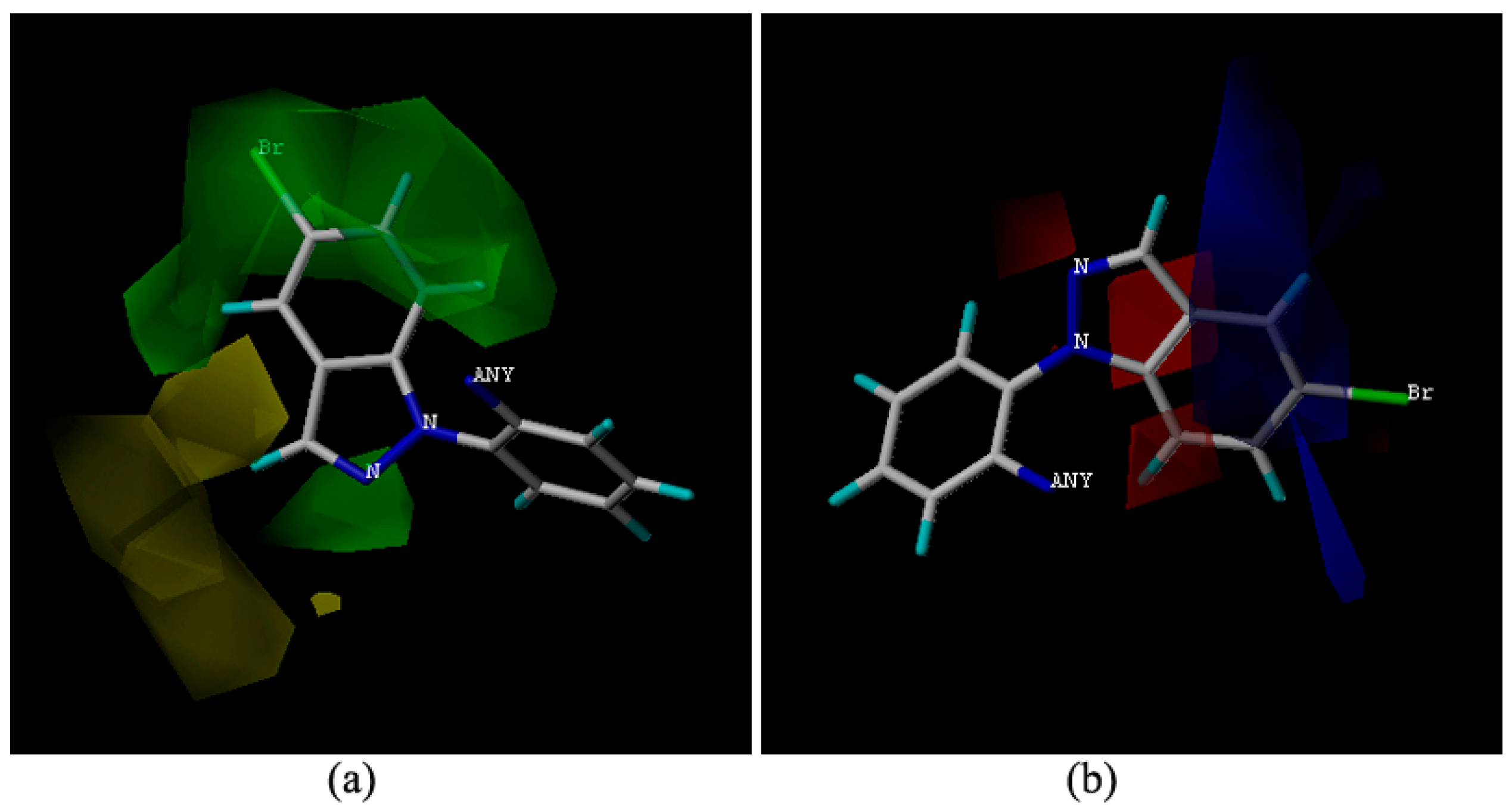
2.4. Molecular Docking

3. Experimental Section
3.1. General Information
3.2. Synthesis of Compounds
3.2.1. Synthesis of 2-(2-Nitrophenyl)benzo[d]thiazole (1)
3.2.2. Synthesis of 2-(Benzo[d]thiazol-2-yl)anilines 2 and 8a–8m
3.2.3. General Procedure for the Preparation of 2-Aryl-3-aminopyridines 5a–5g
3.2.4. General Procedures for the Preparation of 2-Substituted Aminoanilines 3a, 6a–6g and 9a–9n
3.3. Bioassays
3.4. QSAR Analyses
3.5. Molecular Docking
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Angelini, R.M.; Masiello, M.; Rotolo, C.; Pollastro, S.; Faretra, F. Molecular characterisation and detection of resistance to succinate dehydrogenase inhibitor fungicides in Botryotinia fuckeliana (Botrytis cinerea). Pest. Manag. Sci. 2014, 70, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Du, S.J.; Lu, H.Z.; Yang, D.Y.; Li, H.; Gu, X.L.; Wan, C.; Jia, C.Q.; Wang, M.; Li, X.Y.; Qin, Z.H. Synthesis, antifungal activity and QSAR of some novel carboxylic Acid amides. Molecules 2015, 20, 4071–4087. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, H.; Gerpe, A.; Gonzalez, M.; Aran, V.J.; de Ocariz, C.O. Pharmacological properties of indazole derivatives: Recent developments. Mini-Rev. Med. Chem. 2005, 10, 869–878. [Google Scholar] [CrossRef]
- Schmidt, A.; Beutler, A.; Snovydovych, B. Recent Advances in the Chemistry of Indazoles. Eur. J. Org. Chem. 2008, 24, 4073–4095. [Google Scholar] [CrossRef]
- Maklashina, E.; Cecchini, G. The quinone-binding and catalytic site of complex II. Biochim. Biophys. Acta 2010, 12, 1877–1882. [Google Scholar] [CrossRef]
- Huang, L.S.; Sun, G.; Cobessi, D.; Wang, A.C.; Shen, J.T.; Tung, E.Y.; Anderson, V.E.; Berry, E.A. 3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme. J. Biol. Chem. 2006, 281, 5965–5972. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, B.A.; Bayon, C.; Atkins, S.; Cools, H.J.; Lucas, J.A.; Fraaije, M.W. Risk assessment studies on succinate dehydrogenase inhibitors, the new weapons in the battle to control Septoria leaf blotch in wheat. Mol. Plant. Pathol. 2012, 13, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhai, Y.; Lou, J.; Liu, M.; Pang, X.; Sun, F. Thiabendazole inhibits ubiquinone reduction activity of mitochondrial respiratory complex II via a water molecule mediated binding feature. Protein Cell. 2011, 2, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, H.; Qu, F.Q.; Huang, X.L. Potassium Ferricyanide Oxidative Cyclizationof Arylaldehyde with o-Phenylenediamine and o-Aminophenol to 2-Arylbenzimidazoles and 2-Arylbenzoxazoles. J. Wuhan Univ. Nat. 1999, 3, 355–357. [Google Scholar]
- Bavin, P.M.G. 2-AMINOFLUORENE. Org. Synth. 1973, 5, 30. [Google Scholar]
- Yadav, V.; Gupta, S.; Kumar, R.; Singh, G.; Lagarkha, R. Polymeric PEG35k-Pd Nanoparticles: Efficient and Recyclable Catalyst for Reduction of Nitro Compounds. Synth. Commun. 2012, 42, 213–222. [Google Scholar] [CrossRef]
- Caron, S.; Massett, S.S.; Bogle, D.E.; Castaldi, M.J.; Braish, T.F. An efficient and cost-effective synthesis of 2-phenyl-3-aminopyridine. Org. Process Res. Dev. 2001, 5, 254–256. [Google Scholar] [CrossRef]
- Pritchard, G.; Bowman, W.; Lyon, J. Palladium-Mediated Synthesis of Phenanthridines: The First Report of Palladium Insertion into Imidoyl Selenides. Synlett 2008, 14, 2169–2171. [Google Scholar] [CrossRef]
- Tobler, H.; Walter, H.; Ehrenfreund, J.; Corsi, C.; Giordano, F.; Zeller, M.; Seifert, G.; Shah, S.C.; George, N.; Jones, I. Process for the Preparation of Pyrazole Carboxylic Acid Tetrahydro Isopropyl Methanonaphthalen Amides from Isopropylidene Nitrobenzonorbornadienes and Pyrazolecarboxylates. WO Patent 2007031323, 14 September 2006. [Google Scholar]
- Yuan, X.Y.; Zhang, L.; Han, X.Q.; Zhou, Z.Y.; Du, S.J.; Wan, C.; Yang, D.Y.; Qin, Z.H. Synthesis and Fungicidal Activity of the Strobilurins Containing 1,3,4-Thiodiazole Ring. Chin. J. Org. Chem. 2014, 34, 170–177. [Google Scholar] [CrossRef]
- Ding, W.; Sun, M.; Luo, S.; Xu, T.; Cao, Y.; Yan, X.; Wang, Y. A 3D QSAR study of betulinic acid derivatives as anti-tumor agents using topomer CoMFA: Model building studies and experimental verification. Molecules 2013, 18, 10228–10241. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Tian, Z.; Yang, D.; Li, X.; Li, H.; Jia, C.; Che, C.; Wang, M.; Qin, Z. Synthesis, Antifungal Activity and Structure-Activity Relationships of Novel 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid Amides. Molecules 2015, 20, 8395-8408. https://doi.org/10.3390/molecules20058395
Du S, Tian Z, Yang D, Li X, Li H, Jia C, Che C, Wang M, Qin Z. Synthesis, Antifungal Activity and Structure-Activity Relationships of Novel 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid Amides. Molecules. 2015; 20(5):8395-8408. https://doi.org/10.3390/molecules20058395
Chicago/Turabian StyleDu, Shijie, Zaimin Tian, Dongyan Yang, Xiuyun Li, Hong Li, Changqing Jia, Chuanliang Che, Mian Wang, and Zhaohai Qin. 2015. "Synthesis, Antifungal Activity and Structure-Activity Relationships of Novel 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid Amides" Molecules 20, no. 5: 8395-8408. https://doi.org/10.3390/molecules20058395
APA StyleDu, S., Tian, Z., Yang, D., Li, X., Li, H., Jia, C., Che, C., Wang, M., & Qin, Z. (2015). Synthesis, Antifungal Activity and Structure-Activity Relationships of Novel 3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic Acid Amides. Molecules, 20(5), 8395-8408. https://doi.org/10.3390/molecules20058395





