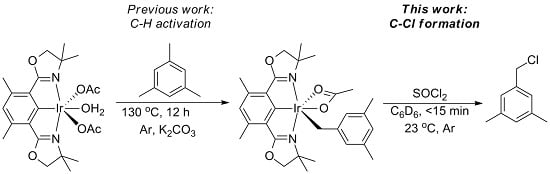
Chlorination of (Phebox)Ir(mesityl)(OAc) by Thionyl Chloride
Abstract
:1. Introduction

2. Results and Discussion



3. Experimental Section
3.1. General
3.2. Chlorination of Complex 2
3.3. Syntheses and Characterizations




4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, X.; Shan, G.; Sun, Y.; Rao, Y. Regio- and Chemoselective C-H Chlorination/Bromination of Electron-Deficient Arenes by Weak Coordination and Study of Relative Directing-Group Abilities. Angew. Chem. Int. Ed. 2013, 52, 4440–4444. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, R.; Qiao, X.; Shen, Z. Copper-Catalyzed Aromatic C-H Bond Halogenation Using Lithium Halides as Halogenating Reagents. Synlett 2011, 2011, 1038–1042. [Google Scholar] [CrossRef]
- Molander, G.A.; Elia, M.D. Suzuki-Miyaura Cross-Coupling Reactions of Benzyl Halides with Potassium Aryltrifluoroborates. J. Org. Chem. 2006, 71, 9198–9202. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. The Organometallic Chemistry of Alkanes. Chem. Rev. 1985, 85, 245–269. [Google Scholar] [CrossRef]
- Crabtree, R.H. Organometallic Alkane CH Activation. J. Organomet. Chem. 2004, 689, 4083–4091. [Google Scholar] [CrossRef]
- Bergman, R.G. Organometallic chemistry—C-H activation. Nature 2007, 446, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.R.; Sanford, M.S. Transition Metal Catalyzed Oxidative Functionalization of Carbon–hydrogen Bonds. Tetrahedron 2006, 62, 2439–2463. [Google Scholar] [CrossRef]
- Stowers, K.J.; Sanford, M.S. Mechanistic Comparison between Pd-Catalyzed Ligand-Directed C-H Chlorination and C-H Acetoxylation. Org. Lett. 2009, 11, 4584–4587. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, S.R.; Sanford, M.S. Reactivity of Pd(II) Complexes with Electrophilic Chlorinating Reagents: Isolation of Pd(IV) Products and Observation of C-Cl Bond-Forming Reductive Elimination. J. Am. Chem. Soc. 2007, 129, 15142–15143. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, P.; Alla, S.K.; Punniyamurthy, T. Pd(II)-Catalyzed Aminotetrazole-Directed Ortho-Selective Halogenation of Arenes. J. Org. Chem. 2013, 78, 6104–6111. [Google Scholar] [CrossRef] [PubMed]
- Bedford, R.B.; Haddow, M.F.; Mitchell, C.J.; Webster, R.L. Mild C-H Halogenation of Anilides and the Isolation of an Unusual Palladium(I)–Palladium(II) Species. Angew. Chem. Int. Ed. 2011, 50, 5524–5527. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Jiang, X.; Sun, P. Palladium-Catalyzed Highly Selective ortho-Halogenation (I, Br, Cl) of Arylnitriles via sp2 C-H Bond Activation Using Cyano as Directing Group. J. Org. Chem. 2013, 78, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, N.; Schröder, N.; Glorius, F. Rh(III)-Catalyzed Halogenation of Vinylic C-H Bonds: Rapid and General Access to Z-Halo Acrylamides. Org. Lett. 2013, 15, 3860–3863. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhou, X.; Pu, S.; Cao, B. Rhodium-catalyzed ortho-selective C-H halogenation of 2-arylbenzo[d]thiazoles using N-halosuccinimides as halogen sources. Tetrahedron 2015, 71, 2376–2381. [Google Scholar] [CrossRef]
- Liu, W.; Groves, J.T. Manganese Porphyrins Catalyze Selective C-H Bond Halogenations. J. Am. Chem. Soc. 2010, 132, 12847–12849. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Balcells, D.; Parent, A.R.; Crabtree, R.H.; Eisenstein, O. Cp* Iridium Precatalysts for Selective C-H Oxidation via Direct Oxygen Insertion: A Joint Experimental/Computational Study. ACS Catal. 2011, 2, 208–218. [Google Scholar] [CrossRef]
- Zhou, M.; Hintermair, U.; Hashiguchi, B.G.; Parent, A.R.; Hashmi, S.M.; Elimelech, M.; Periana, R.A.; Brudvig, G.W.; Crabtree, R.H. Cp* Iridium Precatalysts for Selective C-H Oxidation with Sodium Periodate as the Terminal Oxidant. Organometallics 2013, 32, 957–965. [Google Scholar] [CrossRef]
- Zhou, M.; Schley, N.D.; Crabtree, R.H. Cp* Iridium Complexes Give Catalytic Alkane Hydroxylation with Retention of Stereochemistry. J. Am. Chem. Soc. 2010, 132, 12550–12551. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kwak, J.; Shin, K.; Lee, D.; Chang, S. Ir(III)-Catalyzed Mild C-H Amidation of Arenes and Alkenes: An Efficient Usage of Acyl Azides as the Nitrogen Source. J. Am. Chem. Soc. 2013, 135, 12861–12868. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.-I.; Kaneda, T.; Nishiyama, H. Intermolecular C-H Bond Activation of Alkanes and Arenes by NCN Pincer Iridium(III) Acetate Complexes Containing Bis(oxazolinyl)phenyl Ligands. Organometallics 2012, 31, 4442–4449. [Google Scholar] [CrossRef]
- Allen, K.E.; Heinekey, D.M.; Goldman, A.S.; Goldberg, K.I. Regeneration of an Iridium(III) Complex Active for Alkane Dehydrogenation Using Molecular Oxygen. Organometallics 2014, 33, 1337–1340. [Google Scholar] [CrossRef]
- Pahls, D.R.; Allen, K.E.; Goldberg, K.I.; Cundari, T.R. Understanding the Effect of Ancillary Ligands on Concerted Metalation-Deprotonation by (dmPhebox)Ir(OAc)2(H2O) Complexes: A DFT Study. Organometallics 2014, 33, 6413–6419. [Google Scholar] [CrossRef]
- Allen, K.E.; Heinekey, D.M.; Goldman, A.S.; Goldberg, K.I. Alkane Dehydrogenation by C-H Activation at Iridium(III). Organometallics 2013, 32, 1579–1582. [Google Scholar] [CrossRef]
- Owens, C.P.; Varela-Alvarez, A.; Boyarskikh, V.; Musaev, D.G.; Davies, H.M.L.; Blakey, S.B. Iridium(iii)-bis(oxazolinyl)phenyl Catalysts for Enantioselective C-H Functionalization. Chem. Sci. 2013, 4, 2590–2596. [Google Scholar] [CrossRef]
- Zhou, M.; Johnson, S.I.; Yang, G.; Emge, T.J.; Nielsen, R.J.; Goddard, W.A., III; Goldman, A.S. Activation and Oxidation of Mesitylene C-H Bonds by (Phebox)Ir(III) Complexes. Organometallics 2015. [Google Scholar] [CrossRef]
- Ito, J.-I.; Shiomi, T.; Nishiyama, H. Efficient Preparation of New Rhodium- and Iridium-[Bis(oxazolinyl)-3,5-dimethylphenyl] Complexes by C-H Bond Activation: Applications in Asymmetric Synthesis. Adv. Synth. Catal. 2006, 348, 1235–1240. [Google Scholar] [CrossRef]
- Schmid, G.; Ritter, G. Versuche zur Oxydativen Addition von Thionylhalogeniden an Übergangsmetallkomplexe. Z. Anorg. Allg. Chem. 1975, 415, 97–103. [Google Scholar] [CrossRef]
- Markham, S.J.; Chung, Y.L.; Branum, G.D.; Blake, D.M. Reactions of Iridium(I) Compounds with Oxidized Derivatives of Organic Disulfides and with Thionyl Chloride. J. Organomet. Chem. 1976, 107, 121–127. [Google Scholar] [CrossRef]
- Cartwright, J.; Hill, A.F. Oxidative Addition of Seleninyl Chloride to Vaska’s Complex. Polyhedron 1996, 15, 157–159. [Google Scholar] [CrossRef]
- Vanderpool, R.A.; Abrahamson, H.B. Reaction of Vaska’s complex with thionyl chloride. Inorg. Chem. 1985, 24, 2985–2989. [Google Scholar] [CrossRef]
- Brewster, T.P.; Blakemore, J.D.; Schley, N.D.; Incarvito, C.D.; Hazari, N.; Brudvig, G.W.; Crabtree, R.H. An Iridium(IV) Species, [Cp*Ir(NHC)Cl]+, Related to a Water-Oxidation Catalyst. Organometallics 2011, 30, 965–973. [Google Scholar] [CrossRef]
- Isobe, K.; Bailey, P.M.; Maitlis, P.M. An Iridium(V) Organometallic Compound; Synthesis and X-ray Crystal Structure of Tetramethyl([small eta]5-pentamethylcyclo-pentadienyl)iridium. J. Chem. Soc. Chem. Commun. 1981, 808–809. [Google Scholar] [CrossRef]
- Lehman, M.C.; Pahls, D.R.; Meredith, J.M.; Sommer, R.D.; Heinekey, D.M.; Cundari, T.R.; Ison, E.A. Oxyfunctionalization with Cp*IrIII(NHC)(Me)(Cl) with O2: Identification of a Rare Bimetallic IrIV μ-Oxo Intermediate. J. Am. Chem. Soc. 2015, 137, 3574–3584. [Google Scholar] [CrossRef] [PubMed]
- Hay-Motherwell, R.S.; Wilkinson, G.; Hussain-Bates, B.; Hursthouse, M.B. Synthesis and X-ray Crystal Structure of Oxotrimesityliridium(V). Polyhedron 1993, 12, 2009–2012. [Google Scholar] [CrossRef]
- Lehman, M.C.; Boyle, P.D.; Sommer, R.D.; Ison, E.A. Oxyfunctionalization with Cp*IrIII(NHC)(Me)L Complexes. Organometallics 2014, 33, 5081–5084. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Goldman, A.S. Chlorination of (Phebox)Ir(mesityl)(OAc) by Thionyl Chloride. Molecules 2015, 20, 10122-10130. https://doi.org/10.3390/molecules200610122
Zhou M, Goldman AS. Chlorination of (Phebox)Ir(mesityl)(OAc) by Thionyl Chloride. Molecules. 2015; 20(6):10122-10130. https://doi.org/10.3390/molecules200610122
Chicago/Turabian StyleZhou, Meng, and Alan S. Goldman. 2015. "Chlorination of (Phebox)Ir(mesityl)(OAc) by Thionyl Chloride" Molecules 20, no. 6: 10122-10130. https://doi.org/10.3390/molecules200610122
APA StyleZhou, M., & Goldman, A. S. (2015). Chlorination of (Phebox)Ir(mesityl)(OAc) by Thionyl Chloride. Molecules, 20(6), 10122-10130. https://doi.org/10.3390/molecules200610122