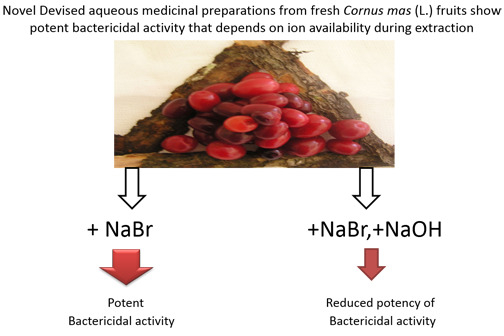
Cornus mas (Linnaeus) Novel Devised Medicinal Preparations: Bactericidal Effect against Staphylococcus aureus and Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cornus mas L. Medicinal Preparations


| Tested Solution | Initial Inoculum cfu/mL | Day 7 Average a Value (SD b) | Day 14 Average Value (SD) | Day 21 | Day 28 | SecondInoculum cfu/mL c | Day 42 Average Value (SD) | Day 49 |
|---|---|---|---|---|---|---|---|---|
| A.A.L.E.P | 5 × 105 | 1.3 × 103 (2 × 102) | 1.8 × 102 (3 × 10) | <10 | <10 | 5 × 105 | 2.2 × 102 (40) | <10 |
| A.O.E.P | 5 × 105 | <10 | <10 | <10 | <10 | 5 × 105 | <10 | <10 |
| Extract in PBS | 5 × 105 | >107 | NMC d | NMC | NMC | - | - | - |
| Extract in ddH2O | 5 × 105 | NMC | NMC | NMC | NMC | - | - | - |
| Methanol Extracts | 5 × 105 | NMC | NMC | NMC | NMC | - | - | -- |
| PBS/ddH2O | 5 × 105 | NMC | NMC | NMC | NMC | - | - | - |
| 100 mM NaBr | 5 × 105 | 2 × 106 | >108 | NMC | NMC | - | - | - |
| 200 mM NaBr | 5 × 105 | 1.8 × 106 | >108 | NMC | NMC | - | - | - |
| Extract in PBS & Extract in ddH2O + 3 g/L octanediol | 5 × 105 | <10 | <10 | <10 | <10 | - | - | - |
| Tested Solution | Initial Inoculum cfu/mL | Day 7 Average a Value (SD b ) | Day 14 | Day 21 | Day 28 | SecondInoculumcfu/mL c | Day 42 | Day 49 |
|---|---|---|---|---|---|---|---|---|
| A.A.L.E.P | 2 × 105 | 1.3 × 102 (3× 10) | <10 | <10 | <10 | 2 × 105 | <10 | <10 |
| A.O.E.P | 2 × 105 | <10 | <10 | <10 | <10 | 2 × 105 | <10 | <10 |
| Extract in PBS | 2 × 105 | >106 | NMC | NMC | NMC | - | - | - |
| Extract in ddH2O | 2 × 105 | NMC d | NMC | NMC | NMC | - | - | - |
| Methanol Extracts | 2 × 105 | NMC | NMC | NMC | NMC | - | - | - |
| PBS/ddH2O | 2 × 105 | NMC | NMC | NMC | NMC | - | - | - |
| 100 mMNaBr | 2 × 105 | 4 ×106 (2 × 106) | NMC | NMC | NMC | - | - | - |
| 200 mM NaBr | 2 × 105 | 2 × 105 (2 × 105) | NMC | NMC | NMC | - | - | - |
| Extract in PBS & Extract in ddH2O + 3 g/L octanediol | 2 × 105 | <10 | <10 | <10 | <10 | - | - | - |

2.1.1. Aqueous Alkaline Lysis Extraction Procedure (A.A.L.E.P)
2.1.2. Aqueous Osmotic Extraction Procedure (A.O.E.P)
2.2. Bactericidal Effect
2.3. Cornus mas L. Fruit Nutritional Elements
Favorable Conditions by the Novel Methodology to Generate Stable Compounds



2.4. Selective Antimicrobial Inhibition
Relevance to the Antimicrobial Resistance Trends of S. aureus and P. aeruginosa
| Tested Solution | Initial Inoculum cfu/mL | Day 7 Average Value a (SD b) | Day 14 Average Value (SD) | Day 21 Average Value (SD) | Day 28 Average Value (SD) |
|---|---|---|---|---|---|
| A.A.L.E.P | 1.6 × 105 | 1.5 × 105 (4 × 104) | 1.5 × 106 (5 × 105) | 8.9 × 106 (9 × 105) | 8 × 108 (9 × 107) |
| A.O.E.P | 1.6 × 105 | 1.8 × 105 (6 × 104) | 2× 106 (8 × 105) | 2.2× 106 (8 × 105) | 2 × 108 (1.1 × 107) |
| Extract in PBS | 1.6 × 105 | 2 × 106 (1 × 105) | NMC c | NMC | NMC |
| Extract in ddH2O | 1.6 × 105 | NMC | NMC | NMC | NMC |
| Methanol Extracts | 1.6 × 105 | NMC | NMC | NMC | NMC |
| PBS/ddH2O | 1.6 × 105 | NMC | NMC | NMC | NMC |
| 100 mM and 200 mM NaBr | 1.6 × 105 | 2 × 106 (1 × 105) | NMC | NMC | NMC |
| Extract in PBS and Extract in ddH2O + 3 g/L octanediol | 1.6× 105 | <10 | <10 | <10 | <10 |
3. Experimental Section
3.1. Materials
3.1.1. Plant Material
3.1.2. Extraction Material
3.1.3. Microbiology Material & Equipment
3.2. Methods
3.2.1. A.A.L.E.P.
3.2.2. A.O.E.P.
3.2.3. PBS Extraction
3.2.4. ddH2O Extraction
3.2.5. Methanol Extractions
3.2.6. PBS
3.2.7. ddH2O
3.2.8. 100 mM NaBr and 200 mM NaBr
3.2.9. Extract in PBS with 3 g/L 1,2-octanediol and Extract in ddH2O with 3 g/L 1,2-octanediol
3.2.10. Antimicrobial Preservative Effectiveness Test Category 2
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- He, L.H. Comparative study for α-glucosidase inhibitory effects of total iridoid glycosides in the crude products and the wine-processed products from Cornus officinalis. Yakugaku Zasshi 2011, 131, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Asquary, S. Biochemical and histopathological study of the anti-hyperglycemic and anti-hyperlipidemic effects of cornelian cherry (Cornus mas L.) in alloxan-induced diabetic rats. J. Complement. Integr. Med. 2014, 11, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Alavian, S.M.; Banihabib, M.; Haghi, M.E.; Panahi, F. Protective effect of Cornus mas fruits extract on serum biomarkers in CCl4-induced hepatotoxicity in male rats. Hepat. Mon. 2014, 14, e10330. [Google Scholar] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalana, J.; Bielskad, K.; Merwid-Ląda, A.; Woźniake, A.; Dzimiraf, S.; Pióreckig, N.; Trochaa, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in atherosclerotic rabbits. Phytomedicine 2014, 2113, 1774–1784. [Google Scholar]
- Jayaprakasam, Β.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthrocyanines and ursolic acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.; Koukourikou, M.; Sotiropoulos, T.; Stylianidis, D. Antioxidant activity of fruits produced in northern Greece. Hortscience 2010, 45, 1341–1344. [Google Scholar]
- Bushra, S.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Bushra, S.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Molbovan, B.; David, L. Influence on temperature and preserving agents on the stability of cornelian cherries anthocyanins. Molecules 2014, 19, 8177–8188. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, A.M.; Mariou-panagioti, A.; Nikolaou, T.D.; Georgiou, L.K. Aqueous Acid Preparation Used for Soft Alkaline Lysis Extraction and Biochemical Treatment of the Cornus mascula Fresh Fruit Nutritional Elements. Patent Granted: Appl. No: GR20120100560, Patent No: GR1008036, International Patent Classification: C12M1/00. Category: A, 7 November 2012. [Google Scholar]
- Kyriakopoulos, A.M. Strong Antimicrobial-Action Preparation Derived from the Biochemical Treatment of the Fresh Cornus mascula Fruit Pulp with Sodium Bromide for Acting Against Pseudomonas aeruginosa and Staphylococcus aureus. Patent Granted: Appl. No: GR20130100569, Patent No: GR1008363, International Classification No: A 61K 36/00 Category: A, 7 October 2012. [Google Scholar]
- Vu, N.; Nguyen, K.; Kupiec, T.C. The essentials of United States Pharmacopeia Chapter 51: Antimicrobial effectiveness testing and its applications in pharmaceutical compounding. Int. J. Pharm. Comd. 2014, 18, 123–130. [Google Scholar]
- Cindric, J.J.; Zeiner, M.; Stingeder, J. ICP-AES determination of minor and major elements in Cornellian Cherry (Cornus mas L.) after microwave assisted digestion. Microchem. J. 2012, 105, 72–76. [Google Scholar] [CrossRef]
- Rudrapaul, P.; Kyriakopoulos, A.M.; De, U.C.; Zoumpourlis, V.; Dinda, B. New flavonoids from the fruits of Cornus mas, Cornaceae. Phytochem. Lett. 2015, 11, 292–295. [Google Scholar] [CrossRef]
- Binutu, O.A.; Cordell, G.A. Constituents of Afzelia bella stem bark. Phytochemistry 2001, 56, 827–830. [Google Scholar] [CrossRef]
- Choudhury, R.; Chowrimootoo, G.; Srai, K.; Debnam, E.; Rice-Evans, C.A. Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem. Biophys. Res. Commun. 1999, 265, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; Bilia, A.R.; Mendez, J.; Morelli, I. Myricetin glycosides from Licania densiflora. Fitoterapia 2001, 72, 182–185. [Google Scholar] [CrossRef]
- Elmarie, W.; Pretorius, J.C. Purification and identification of active antibacterial components in Carpobrotus edulis L. J. Ethnopharmacol. 2001, 76, 87–91. [Google Scholar]
- Nara, S.L.; Harjani, J.R.; Salukhe, A.M. Lipase-catalysed transesterification in ionic liquids and organic solvents: A comparative study. Tetrahedron Lett. 1992, 43, 2979–2982. [Google Scholar] [CrossRef]
- Avent, A.G.; Chaloner, P.A.; Day, M.P.; Seddon, K.R.; Welton, T. Evidence for hydrogen bonding in solutions of 1-ethyl-3-methylimidazolium halides, and its implications for room temperature halogenoaluminate (III) ionic liquids. J. Chem. Soc. Dalton Trans. 1994. [Google Scholar] [CrossRef]
- O’Neil, M.J. The Merck Index-An Encyclopedia of Chemicals, Drugs, and Biologicals; Merck and Co., Inc.: Whitehouse Station, NJ, USA, 2006; p. 1480. [Google Scholar]
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally occuring iridoids. A Review, Part 1. Chem. Pharm. Bull. 2007, 55, 159–222. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.W. Oligosaccharides; Pergamon Press: Oxford, UK, 1965. [Google Scholar]
- Dawder, J.G.; Moore, A.T. The Structure and Properties of Some Natural Organic Compounds. In Chemistry for the Life Sciences; Palgrave Macmillan: London, UK, 1980. [Google Scholar]
- Zhong, Z.; Snowden, T.S.; Best, M.D.; Anslyn, E.V. Rate of enolate formation is not very sensitive to the hydrogen bonding ability of donors to carboxyl oxygen lone pair acceptors; a ramification of the principle of non-perfect synchronization for general-base-catalyzed enolate formation. J. Am. Chem. Soc. 2004, 126, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Gavin, T.; Geohagen, B.C.; Zhang, L.; Casper, D.; Lekhraj, R.; Barder, D.S. Β-dicarbonyl enolates: A new class of neuroprotectants. J. Neurochem. 2011, 116, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, R.; Dubois, J.E. Mechanism of the structural transformations of anthrocyanines in acidic media. J. Am. Chem. Soc. 1997, 99, 1359–1364. [Google Scholar] [CrossRef]
- Hrazdina, G. Anthocyanins. In The Flavonoids: Advances in Research; Harborne, J.B., Mabry, T.J., Eds.; Chapman and Hall: London, UK, 1982; pp. 135–188. [Google Scholar]
- Yaday, P.; Parshad, B.; Manchanda, P.; Sharma, S.K. Chromones and their derivatives as radical scavengers: A remedy for cell impairment. Curr. Top Med. Chem. 2014, 14, 2552–2575. [Google Scholar]
- Peng, L.; Wang, B.; Ren, P. Reduction of MTT by Flavonoids in the absence of cells. Colloids Surf. B Biointerfaces 2005, 45, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Roehri-Stoeckel, C.; Gonzalez, E.; Fougerousse, A.; Brouillard, R. Synthetic dyes: Simple and original ways to 4-substituted flavylium salts and their corresponding vitisin derivatives. Can. J. Chem. 2001, 79, 1173–1178. [Google Scholar] [CrossRef]
- Olszanecki, R.; Kurnyta, M.; Biedron, R.; Chorobik, P.; Bereta, M.; Marcinkiewicz, J. The role of heme oxygenase-1 in down regulation of PGE2 production by taurine chloramine and taurine bromamine in J774.2 macrophages. Amino Acids 2008, 35, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Bozeman, P.M.; Jefferson, M.M.; King, C.C. Oxidation of bromide by the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. Formation of bromamines. J. Biol. Chem. 1995, 270, 2906–2913. [Google Scholar] [PubMed]
- Marcinciewich, J.; Chain, B.; Nowak, B.; Grabowska, A.; Bryniarski, K.; Baran, J. Antimicrobial and cytotoxic activity of hypochlorous acid: Interactions with taurine and nitrite. Inflamm. Res. 2000, 49, 280–289. [Google Scholar] [CrossRef]
- Marcinciewich, J.; Mak, M.; Bobek, M.; Biedroń, R.; Białecka, A.; Koprowski, M.; Kontny, E.; Maśliński, W. Is there a role of taurine bromamine in inflammation? Interactive effects with nitrite and hydrogen peroxide. Inflamm. Res. 2005, 54, 42–49. [Google Scholar]
- Nagl, M.; Hess, M.W.; Pfaller, K.; Hengster, P.; Gottardi, W. Bactericidal activity of micromolar N-chlorotaurine: Evidence for its antimicrobial function in the human defence system. Antimicrob. Agents Chemother. 2000, 44, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Kuzminov, A. The precarious prokaryotic chromosome. J. Bacteriol. 2014, 196, 1793–1806. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.J.; Hitchcock, C.A.; Sibley, C.M. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 1999, 12, 40–79. [Google Scholar]
- Bambeke, F.V.; Glupczinski, Y.; Plesiat, P.; Pechere, J.C.; Tulkens, P.M. Antibiotic efflux pumps in prokaryotic cells: Occurrence, impact on resistance and strategies for the future of antimicrobial therapy. J. Antim. Chem. 2003, 51, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Waxman, D.J.; Strominger, J.L. Beta-Lactam Antibiotics: Biochemical Modes of Action. In Chemistry and Biology of Beta-Lactam Antibiotics; Morin, R.B., Gorman, M., Eds.; Academic Press: San Diego, CA, USA, 1982; p. 210. [Google Scholar]
- Hooper, D.C.; Wolfson, J.S. The fluoroquinolones: Pharmacology, clinical uses and toxicities in humans. Antimicrob. Agents Chemother. 1985, 28, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Neu, C.H.; Goods, T.D. Antimicrobial Chemotherapy. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas: Galveston, TX, USA, 1996. [Google Scholar]
- Kirby, W. Extraction of a highly potent penicillin inactivator from penicillin resistant Staphylococci. Science 1944, 99, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Barber, M. Methicillin resistant staphylococci. J. Clin. Pathol. 1961, 14, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.L.; Pearson, M.L.; Wilcox, K.R.; Cruz, C.; Lancaster, M.V.; Robinson-Dunn, B.; Tenover, F.C.; Zervos, M.J.; Band, J.D.; White, E.; et al. Emergence of vancomycin resistance in Staphylococcus aureus. New Engl. J. Med. 1999, 340, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Champers, H.F.; DeLeo, F.R. Waves of resistance: Stahylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 621–649. [Google Scholar] [CrossRef] [PubMed]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Lister, P.D.; Wolster, D.L.; Hanson, N.D. Antimicrobial-resistant Pseudomonas auruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed]
- Lampert, P.A. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J. R. Soc. Med. 2002, 95, 22–26. [Google Scholar]
- Grundmann, H.; Aires-de-Sousa, M.; Boyce, J.; Tiemersam, E. Emergence and resurgence of methicillin-resistant Staphlococcus aureus as a public-health threat. Lancet 2006, 368, 874–875. [Google Scholar] [CrossRef]
- Pinto, A.N.; Seth, R.; Zhou, F.; Tallon, J.; Dempsey, K.; Tracy, M.; Gilbert, G.L.; O’Sullivan, M.V.N. Emergence and control of an outbreak of infections due to Panton-Valentine leukocidin positive, ST22 methicillin resistant Staphhylococcus aureus in a neonatal intensive care unit. Clin. Microbiol. Infect. 2013, 19, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Wolter, D.J.; Khalaf, D.; Robledo, I.E.; Vazquez, G.J.; Sante, M.I.; Aquino, E.E.; Goering, R.V.; Hanson, N.D. Surveillance of carbapenem-resistant Pseudomonas aeruginosa isolates from Puerto Rican medical center hospitals: Dissemination of KPC and IMP-18 beta-lactamases. Antimicrob. Agents Chemother. 2009, 53, 1660–1664. [Google Scholar]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijinders, B.A.J.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.; Ind, P.W.; Thomas, C.; Goonesekera, S.; R Haffenden, R.; Abdolrasouli, A.; Fiorentino, F.; Shiner, R.J. Microbial contamination of domiciliary nemubilisers and clinical implications in chronic obstructive pulmonary disease. BMJ Open Resp. Res. 2014, 1, e000018. [Google Scholar] [CrossRef] [PubMed]
- Kon, K.V.; Rai, M.K. Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert Rev. Anti-Infect. Ther. 2012, 10, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Smitha, M.S.; Singh, S.P. The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotechnol. 2014, 14, 4745–4756. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W., Jr.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of isoparaffins as used in cosmetics. Int. J. Toxicol. 2012, 31, 269S–295S. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the medicinal preparations: A.A.L.E.P. and A.O.E.P. as well as control preparations are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakopoulos, A.M.; Dinda, B. Cornus mas (Linnaeus) Novel Devised Medicinal Preparations: Bactericidal Effect against Staphylococcus aureus and Pseudomonas aeruginosa. Molecules 2015, 20, 11202-11218. https://doi.org/10.3390/molecules200611202
Kyriakopoulos AM, Dinda B. Cornus mas (Linnaeus) Novel Devised Medicinal Preparations: Bactericidal Effect against Staphylococcus aureus and Pseudomonas aeruginosa. Molecules. 2015; 20(6):11202-11218. https://doi.org/10.3390/molecules200611202
Chicago/Turabian StyleKyriakopoulos, Anthony M., and Biswanath Dinda. 2015. "Cornus mas (Linnaeus) Novel Devised Medicinal Preparations: Bactericidal Effect against Staphylococcus aureus and Pseudomonas aeruginosa" Molecules 20, no. 6: 11202-11218. https://doi.org/10.3390/molecules200611202
APA StyleKyriakopoulos, A. M., & Dinda, B. (2015). Cornus mas (Linnaeus) Novel Devised Medicinal Preparations: Bactericidal Effect against Staphylococcus aureus and Pseudomonas aeruginosa. Molecules, 20(6), 11202-11218. https://doi.org/10.3390/molecules200611202