Potential Mechanism of Action of 3′-Demethoxy-6-O-demethyl-isoguaiacin on Methicillin Resistant Staphylococcus aureus
Abstract
:1. Introduction

2. Results and Discussion
2.1. Growth Curve of MRSA with Different Concentrations of 3′-Demethoxy-6-O-demethylisoguaiacin

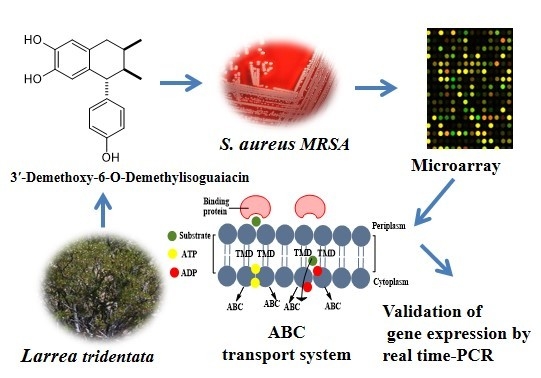
2.2. Microarray Assay and RT-PCR
| Functional Category | Over Expressed Genes (%) | Repressed Genes (%) |
|---|---|---|
| Translation/structural constituent of ribosome | 11.5 | 0 |
| Pathogenesis | 2.5 | 0 |
| Oxidation-reduction process | 3.3 | 0 |
| Metabolic process | 8.3 | 3.6 |
| Unknow function, uncharacterized protein | 14.3 | 37.8 |
| Transcription | 3.3 | 0 |
| Translation | 3.3 | 1.2 |
| Catalytic activity | 2.5 | 0 |
| Biosynthetic process | 7.5 | 8.4 |
| Catabolic process | 2 | 0 |
| Amino acid transport | 2.5 | 0 |
| Transport protein | 0 | 4.8 |
| Glycolisis | 0 | 2.4 |
| DNA repair | 0 | 2.4 |
| Proteolysis | 0 | 2.4 |
| Mechanism of defense | 0 | 4.8 |
| Individual genes with different biological function | 39 | 32.2 |
| Gen ID | Description | Forward 5′→3′ Reverse 5′→3′ | Zscore | RQ | Tm Dissociation |
|---|---|---|---|---|---|
| SAR0144 | ABC transporter ATP-binding protein | GCACTAGAACGGGTCAACA TGGGTCTAATGAAGCAACTGG | −1.507 | −5.922 ± 0.186 | 78.12 |
| SAR1073 | ABC transporter ATP-binding protein | ATGTTGTTTAGAGGGGTCCAC CCAACTTCGCTGCCTACT | −1.834 | −5.780 ± 0.320 | 74.85 |
| SAR1928 | ABC transporter ATP-binding protein | TGAAGTCGTTGCATTTGGAG TCGCTTGGTTACGCATGT | −1.683 | −2.378 ± 0.042 | 76.95 |
| SAR0306 | ABC transporter ATP-binding protein | CGATTGGGTAGGAGGTGTA CCAGAAGGTCCAACTAATGC | −1.782 | −2.039 ±0.307 | 74.91 |
| SAR0618 | Transport system lipoprotein | GAACGCAGTTGGATGTAACC CATACCACAGCCACTCAGAA | −1.917 | −1.392 ±0.061 | 77.04 |
| SAR2267 | FecCD transport family protein | GCGCCTTTATTGGTGGATTA TGAACCAACAAGCCAAAACA | −1.955 | −4.205 ±0.028 | 76.56 |
| SARr016 | 16S ribosomal RNA (Reference gen) | CAGCATGCTACGGTGAATAC GTTACGACTTCACCCCAATC | - | 1 | 76.75 |
3. Experimental Section
3.1. Isolation, Characterization and Evaluation of 3′-Demethoxy-6-O-demethylisoguaiacin
3.2. Growth Inhibitory Curve of MRSA Exposed to Different Concentrations of 3′-Demethoxy-6-O-demethylisoguaiacin
3.3. RNA Isolation and Synthesis of Labeled cDNA
3.4. Microarray and Data Analysis
3.5. Real Time-PCR Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ribeiro Lamblet, L.C.; Aparecida Barbosa, D.D. Prevalence of Staphylococcus aureus colonization in renal transplant patients. Rev. Esc. Enferm. USP 2014, 48, 824–830. [Google Scholar]
- Butterly, A.; Schmidt, U.; Wiener-Kronish, J. Methicillin-resistant Staphylococcus aureus colonization, its relationship to nosocomial infection, and efficacy of control methods. Anesthesiology 2010, 113, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Stryjewski, M.E.; Corey, G.R. Methicillin-resistant Staphylococcus aureus: An evolving pathogen. Clin. Infect. Dis. 2014, 58, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Svarovsky, S.A.; González-Mo, M.J. High-throughput platform for rapid deployment of antimicrobial agents. ACS. Comb. Sci. 2011, 13, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.G.J. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011, 74, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Martínez, J.; Ascacio, J.A.; Rodríguez, R.; Morales, D.; Aguilar, C.N. Phytochemical screening of extracts from some Mexican plants used in traditional medicine. J. Med. Plants Res. 2011, 5, 2791–2797. [Google Scholar]
- Favela-Hernández, J.M.J.; García, A.; Garza-González, E.; Rivas-Galindo, V.M.; Camacho-Corona, M.R. Antibacterial and Antimycobacterial lignans and flavonoids from Larrea tridentata. Phytother. Res. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.J.; Foo, L.; Tokuriki, N.; Afriat, L.; Carr, P.D.; Kim, H.K.; Schenk, G.; Tawfik, D.S.; Ollis, D.L. Conformational sampling, catalysis, and evolution of the bacterial phosphotriesterase. Proc. Natl. Acad. Sci. USA 2009, 106, 21631–21636. [Google Scholar] [CrossRef] [PubMed]
- Seshasayee, A.S.; Fraser, G.M.; Luscombe, N.M. Comparative genomics of cyclic-di-GMP signalling in bacteria: Post-translational regulation and catalytic activity. Nucleic Acids Res. 2010, 38, 5970–5981. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Rubio, A.; Jayaswal, R.K.; Silverman, J.A.; Wilkinson, B.J. Additional routes to Staphylococcus aureus daptomycin resistance as revealed by comparative genome sequencing, transcriptional profiling, and phenotypic studies. PLoS ONE 2013, 8, e58469. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, J.S.; Schroeder, J.W.; Walsh, B.W.; Simmons, L.A. DNA repair and genome maintenance in Bacillus subtilis. Microbiol. Mol. Biol. Rev. 2012, 76, 530–564. [Google Scholar] [CrossRef] [PubMed]
- Rasigade, J.P.; Vandenesch, F. Staphylococcus aureus: A pathogen with still unresolved issues. Infect. Genet. Evol. 2014, 21, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Yi, H.; Chun, J.; Cha, C.J. Genome sequence of type strain of Staphylococcus aureus subsp. aureus. Gut Pathog. 2014, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 2008, 72, 317–364. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, I.M.; Juuti, J.T.; François, P.; Al Majidi, R.; Pietiäinen, M.; Girard, M.; Lindholm, C.; Saller, M.J.; Driessen, A.J.; Kuusela, P.; et al. Inactivation of the Ecs ABC transporter of Staphylococcus aureus attenuates virulence by altering composition and function of bacterial wall. PLoS ONE 2010, 5, e14209. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.P.; Payne, S.M. Vibrio cholerae iron transport systems: Roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect. Immun. 1994, 62, 5120–5125. [Google Scholar] [PubMed]
- Álvarez de Felipe, A.; Pulido-Duarte, M. Transportadores de tipo ABC: Consecuencias de su interacción con flavonoides. Bol. Latinoam. Caribe Plant Med. Aromat. 2008, 7, 296–311. [Google Scholar]
- Zechini, B.; Versace, I. Inhibitors of multidrug resistant efflux systems in bacteria. Anti-Infect. Drug Discov. 2009, 4, 37–50. [Google Scholar] [CrossRef]
- Konno, C.; Xue, H.Z.; Lu, Z.Z.; Ma, B.X.; Erdelmeier, C.A.J.; Che, C.T.; Cordell, G.A.; Soejarto, D.D.; Waller, D.P.; Fong, H.H.S. 1-Aryl Tetralin Lignans from Larrea tridentata. J. Nat. Prod. 1989, 52, 1113–1117. [Google Scholar] [CrossRef]
- Francoeur, A.M.; Assalian, A. Microcat: A novel cell proliferation and cytotoxicity assay based on WST-1. Biochemica 1996, 3, 19–25. [Google Scholar]
- Favela-Hernández, J.M.J. Aislamiento y Caracterización de los Compuestos Antibacterianos y Antituberculosos de Larrea Tridentata, Determinación de su Toxicidad y Mecanismo de Acción del Compuesto Más Activo. Ph.D. Thesis, Facultad de Ciencias Químicas, Universidad Autónoma de Nuevo León, San Nicolás de Los Garza, Mexico, 2012; p. 143. [Google Scholar]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Altermann, E.; Klaenhammer, T.R. PathwayVoyager: Pathway mapping using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. BMC Genomics 2005, 3. [Google Scholar] [CrossRef] [Green Version]
- Meyer, F.; Goesmann, A.; McHardy, A.C.; Bartels, D.; Bekel, T.; Clausen, J.; Kalinowski, J.; Linke, B.; Rupp, O.; Giegerich, R.; et al. GenDB-an open source genome annotation system for prokaryote genomes. Nucleic Acids Res. 2003, 31, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the compound is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Favela-Hernández, J.M.J.; Clemente-Soto, A.F.; Balderas-Rentería, I.; Garza-González, E.; Camacho-Corona, M.D.R. Potential Mechanism of Action of 3′-Demethoxy-6-O-demethyl-isoguaiacin on Methicillin Resistant Staphylococcus aureus. Molecules 2015, 20, 12450-12458. https://doi.org/10.3390/molecules200712450
Favela-Hernández JMJ, Clemente-Soto AF, Balderas-Rentería I, Garza-González E, Camacho-Corona MDR. Potential Mechanism of Action of 3′-Demethoxy-6-O-demethyl-isoguaiacin on Methicillin Resistant Staphylococcus aureus. Molecules. 2015; 20(7):12450-12458. https://doi.org/10.3390/molecules200712450
Chicago/Turabian StyleFavela-Hernández, Juan Manuel J., Aldo F. Clemente-Soto, Isaías Balderas-Rentería, Elvira Garza-González, and María Del Rayo Camacho-Corona. 2015. "Potential Mechanism of Action of 3′-Demethoxy-6-O-demethyl-isoguaiacin on Methicillin Resistant Staphylococcus aureus" Molecules 20, no. 7: 12450-12458. https://doi.org/10.3390/molecules200712450
APA StyleFavela-Hernández, J. M. J., Clemente-Soto, A. F., Balderas-Rentería, I., Garza-González, E., & Camacho-Corona, M. D. R. (2015). Potential Mechanism of Action of 3′-Demethoxy-6-O-demethyl-isoguaiacin on Methicillin Resistant Staphylococcus aureus. Molecules, 20(7), 12450-12458. https://doi.org/10.3390/molecules200712450