Abstract
A series of β-Carboline derivatives were designed, synthesized, and evaluated for their fungicidal activities in this study. Several derivatives electively exhibited fungicidal activities against some fungi. Especially, compound F5 exhibited higher fungicidal activity against Rhizoctonia solani(53.35%) than commercial antiviral agent validamycin (36.4%); compound F16 exhibited high fungicidal activity against Oospora citriaurantii ex Persoon(43.28%). Some of the alkaloids and their derivatives (compounds F4 and F25) exhibited broad-spectrum fungicidal activity. Specifically, compound F4 exhibited excellent high broad-spectrum fungicidal activity in vitro, and the curative and protection activities against P. litchi in vivo reached 92.59% and 59.26%, respectively. The new derivative, F4, with optimized physicochemical properties, obviously exhibited higher activities both in vitro and in vivo; therefore, F4 may be used as a new lead structure for the development of fungicidal drugs.
1. Introduction
Plant pathogenic microorganisms can infect crops, causing local or whole plant disease and leading to significant economic losses. How to control them in modern agriculture is still a big challenge. Many kinds of fungicides are used to prevent and cure the diseases caused by fungi; however, these chemical agents cannot fully protect the crops or completely cure the crops’ tissues from fungal infection under field conditions. Therefore, novel and more practical fungicidal reagents are urgently needed.
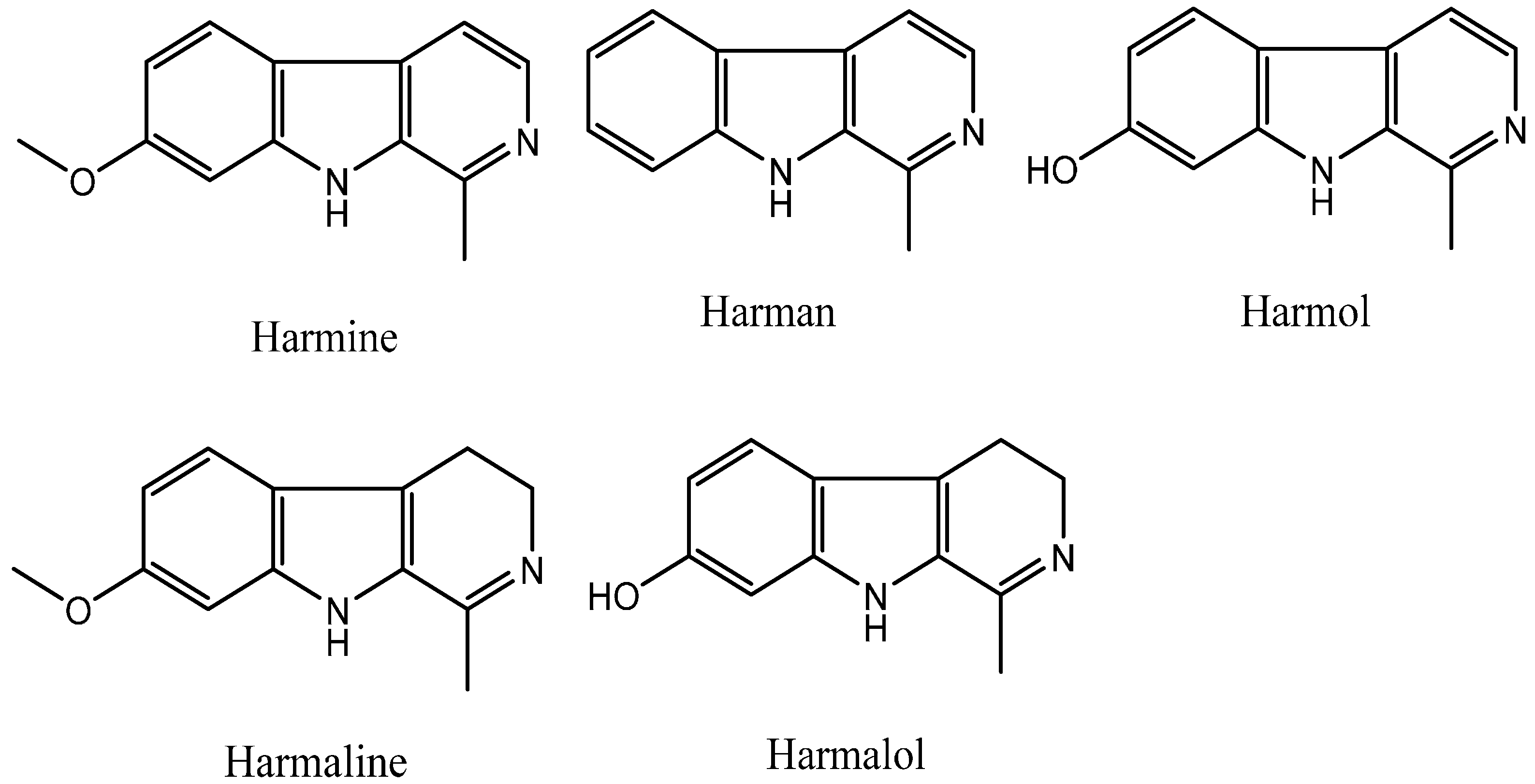
Plants can produce some secondary metabolites with insecticidal, antifungal, or antibacterial biological activity; therefore, natural products can be used as ideal lead structures to develop agrochemicals. The β-carboline alkaloids are a large group of natural and synthetic indole alkaloids that possess a common tricyclic pyrido [3,4-b] indole ring structure (Figure 1) [1,2,3]. Harmine, harman, harmol, harmaline, and harmalol, which are β-carboline and dihydro-β-carboline alkaloids, are four representative harmala alkaloids. Harmine was originally isolated from Peganum harmala L. [4], and found to exhibit a cytotoxic effect on HL60 and K562 leukemic cell lines [5]. Harmane has DNA intercalation ability, leading to not only intercalation into DNA [6,7] and formation DNA adducts [8], but also inhibition of Topo I [7,9], Topo II [9], and MAO-A activity [10,11]. Harmol can induce autophagy and suppression of survivin expression, subsequently induce apoptotic cell death in U251MG human glioma cells [12] and apoptosis by caspase-8 activation independently from Fas/Fas ligand interaction in human non-small cell lung cancer (NSCLC) H596 cells [13], and significantly inhibit the dioxin-mediated induction of CYP1A1 at mRNA, protein, and activity levels in a concentration-dependent manner in human and murine hepatoma cells [14]. Harmaline can inhibit DNA excision repair [15], human DNA Topo I activity [7], PKC activity [16], and TMV [17] and against the amastigote stage of Leishmania [16]. Harmalol is able to induce melanogenesis through p38 MAPK signaling [18] and can act as an agent for preventing dioxin-mediated effects [19].
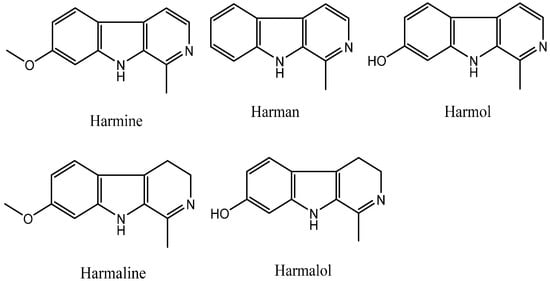
Figure 1.
Chemical structures of β-carboline alkaloids.
Figure 1.
Chemical structures of β-carboline alkaloids.
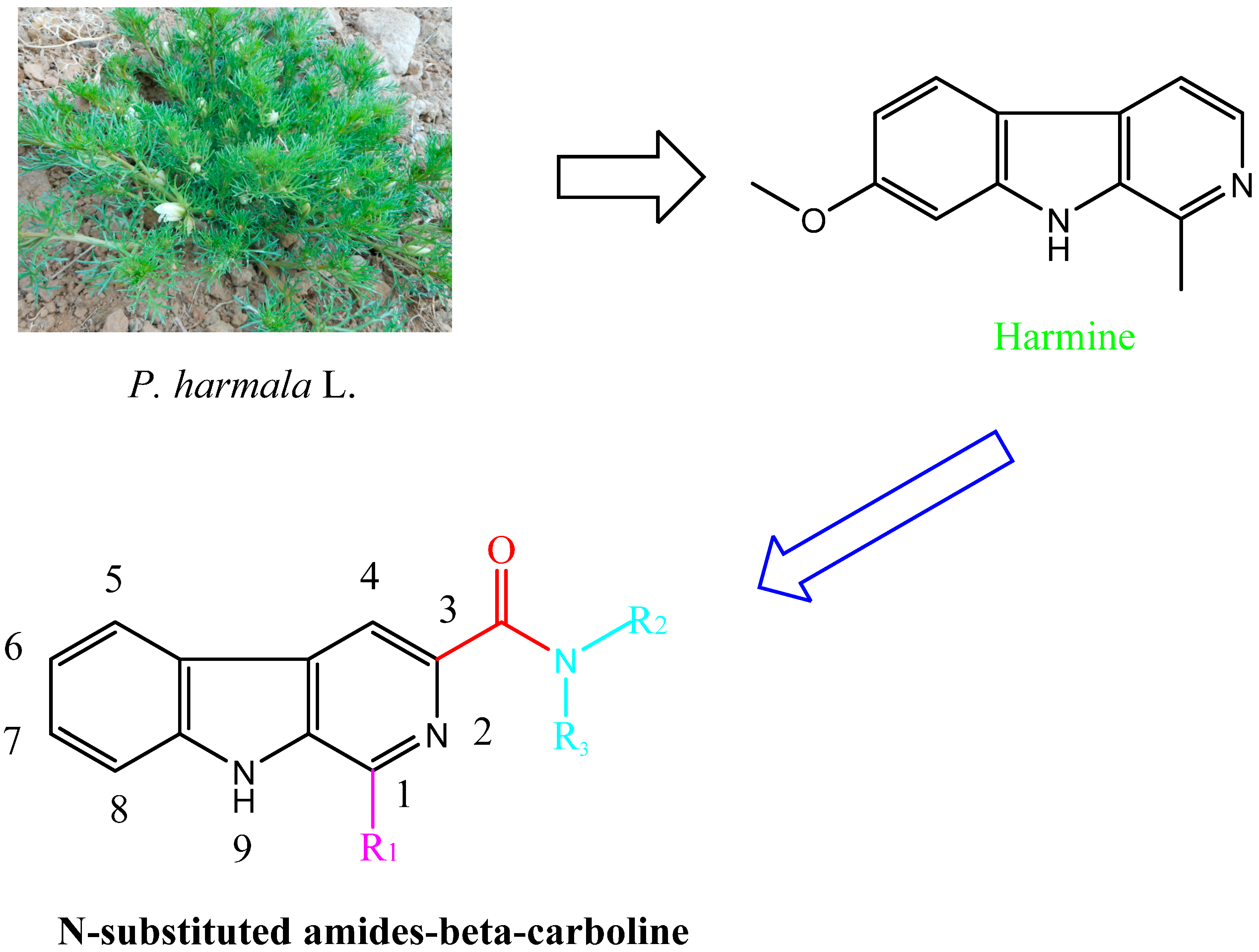
β-Carboline and its structural analogues in the medical and pharmaceutical are a research focus. However, there is limited information about these chemicals in agricultural areas and they lack system development and application. The β-carboline amides, containing amides and a carboline structure, represent a new direction for the development of plant-derived bio-pesticides. In addition, the antifungal activity of β-carboline will change when the 3-position of β-carboline was substituted [17,20]. In this study, their fungicidal activities were systematically evaluated. To investigate the biological activities of the substituents, β-carbolines containing different substituents were synthesized and their fungicidal activities were also systematically evaluated.
2. Results and Discussion
2.1. Synthesis
Compounds harmine, harmane, harmaline, and harmalol were obtained from Sigma-Aldrich, St. Louis, MI, USA. Their chemical structures are shown in Figure 1. Previous structure–activity relationship studies had demonstrated the influence of substituents in positions-1, -3, and -9 of the β-carboline skeleton for a variety of synthetic β-carboline derivatives [21,22,23,24]. In order to study the effect of main structure and the substituent groups at position 1 and 3 on their herbicidal activity, we synthesized a series of 30 novel β-carboline derivatives bearing a substituted amide group at C-3 and substituted groups at C-1 (Figure 2 and Scheme 1). All these compounds were characterized by their melting point, mass, infrared, IR, and 1H-NMR spectra, which confirmed the proposed structures of the new compounds. Tryptophan, which has an electron-rich indole ring, was used as the parent material when applying Pictet–Spengler or Bischler–Napieralski reactions [25] with a variety of aromatic aldehyde cyclization to give tetrahydrocarboline compounds, then oxidizing to obtain β-carboline compounds by using DMF as a solvent and KMnO4 as an oxidant. Pictet–Spengler reactions that used acetic acid as the catalyzed solvent produced a reaction that was refluxed at 80 °C to obtain a higher yield (above 80%) of tetrahydro-β-carboline compounds. Since the reaction temperature was moderate and the by-product generated was less, the product could be obtained with a purity of more than 90% by suction filtration and washing. Then the product could be used directly in the next reaction after drying. The carboxyl on the 3-position of tetrahydro-β-carboline must be protected by esterification, due to the fact that a carboxyl with high reaction activity could be easily decarboxylated, thereby losing carbonyl in the potassium permanganate conditions. Using KMnO4 to oxidize tetrahydro-β-carboline derivatives produced a lower yield, but the reagent was relatively inexpensive, and the reaction was easy to operate in the laboratory. The acylation reaction of amide synthesis used acid halide with ammonia or amine. Step 1: β-carboline-3-carboxylic acid reacted with an excess of thionyl chloride to become the corresponding acid chloride in the situation of catalyzer MDF of 1‰. The excess of thionyl chloride was both reactant and reaction solvent in the reaction, which may also remove water to reduce moisture in the system requirements of dry operation. HCl gas and SO2 gas were generated by the reaction; we utilized lye to absorb them. Step 2: acid chloride reacted with the corresponding amine. The reaction requires adding week base to neutralize the HCl so as to avoid amine reacting with HCl to generate the amine hydrochloride, which does not participate in the reaction. Because the reaction is intense, the product of the first step should be dissolved firstly in methylene chloride, and added slowly dropwise to the mixture of amine and triethylamine that was placed on an ice bath, and then the mixture stirred at room temperature for half an hour.
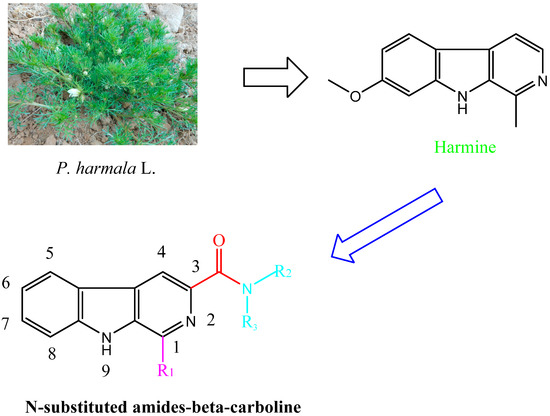
Figure 2.
Design of target compounds.
Figure 2.
Design of target compounds.
2.2. Fungicidal Activities
Fungicide Screening. Compounds F1–30 were evaluated in a series of in vitro fungicidal tests, against a range of phytopathogenic species. The resulting data (Table 1) revealed that these alkaloids and their derivatives displayed potential fungicidal activity against six kinds of plant fungi including F. oxysporum f.sp.cubense, C. gloeosporioides (Penz.), R. solani, P. litchii, P. nicotianae, and O. citriaurantii ex Persoon. When the R1 was phenyl, the compounds exhibited higher activities than those with 4-nitrophenyl, 4-methoxyphenyl,3,4,5-trimethoxyphenyl,4-trifluoromethylphenyl, or 4-chlorophenyl as the R1. When the skeleton was β-carboline, the types of the substituents on the R2 and R3 had a significant influence on the fungicidal activities. For instance, compound F4 (N-(2-pyridyl)-1-phenyl-9H-pyrido[3,4-b]indole-3-formamide) exhibited excellent fungicidal activity against most of the tested fungi, whereas compound F2 (N,N-diethyl-1-phenyl-9H-pyrido[3,4-b]indole-3-formamide) exhibited only moderate activity; compound F5, containing a 4-trifluoromethylphenyl group, exhibited higher fungicidal activity than the compound F1 and the group containing 4-chlorophenyl. Several of these compounds selectively exhibited fungicidal activities against some fungi. For example, the activities of harmine (against F. oxysporum f.sp.cubense and R. solani), compound F16 (against O. citriaurantii ex Persoon), and compound F25 (against C. gloeosporioides (Penz.)) were much higher than that against other fungi and were higher than the other compounds against some fungi, as shown in Table 1.
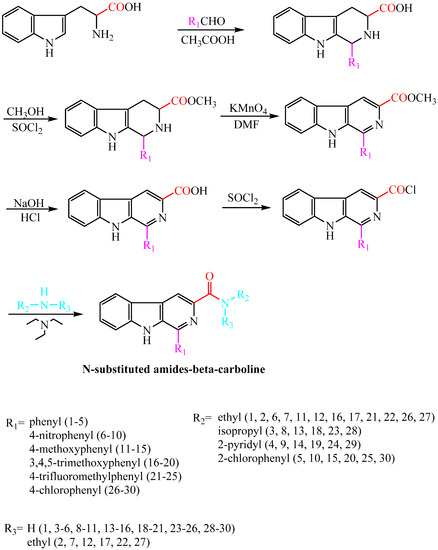
Scheme 1.
Synthesis of target compounds.
Scheme 1.
Synthesis of target compounds.
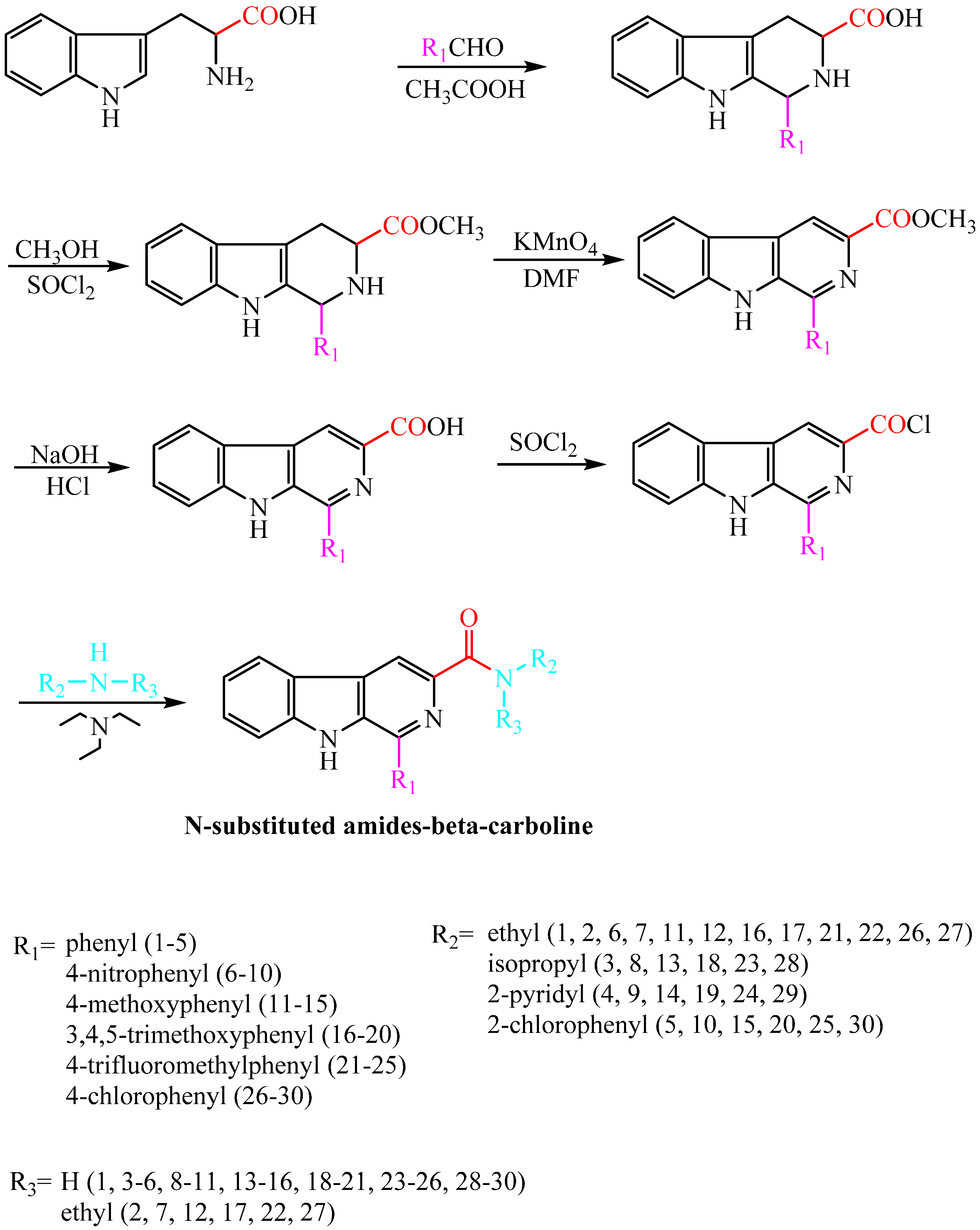

Table 1.
Fungicidal activities of harmine, harmane, harmaline, harmalol, and compounds F1–30 against six kinds of fungi (percent inhibition, %; 100 mg/L).
| Compounds | FO | CG | RS | PL | PN | OC |
|---|---|---|---|---|---|---|
| Harmaline | 32.9 | 19.7 | 38.4 | 49.4 | 30.2 | 10.7 |
| Harmalol | 12.3 | 21 | 25 | 30.4 | 20.4 | 5.8 |
| Harmine | 41.6 | 27.3 | 71.9 | 78.8 | 9.3 | 10.4 |
| Harmol | 33.3 | 13.8 | 27.8 | 46.7 | 38.4 | 10.2 |
| F1 | 23 | 31.5 | 52.9 | 27.4 | 19.5 | 13.5 |
| F2 | 0.2 | 4.6 | 9.9 | - d | - | 0.3 |
| F3 | - | 3.2 | 14.9 | - | 18.6 | - |
| F4 | 30.2 | 32 | 43.2 | 95.9 | 63.3 | 8.5 |
| F5 | 19.9 | 30.5 | 53.4 | 76.3 | 31.7 | 6.8 |
| F6 | 0.5 | 1 | 15.7 | 32.1 | 19 | - |
| F7 | 3.1 | 10.2 | 15.7 | 2.1 | 9.8 | 0.5 |
| F8 | - | 16.5 | 23.8 | 32.4 | 23.6 | - |
| F9 | - | 19.6 | 15.1 | 53 | 36.4 | - |
| F10 | - | 2.8 | 9.1 | 57.7 | 42.4 | 3.4 |
| F11 | 2.8 | 20.1 | 9.4 | 35.6 | 23.4 | 6.8 |
| F12 | 3.4 | 11.4 | 7.3 | 8.2 | 9.6 | - |
| F13 | 9.9 | 11 | 36.6 | / e | / | / |
| F14 | 0.5 | 14 | 18.6 | 50.1 | 11.1 | 2.9 |
| F16 | 7.2 | 7.9 | 31.9 | 3.7 | - | 43.3 |
| F17 | 13.1 | 12.9 | 18.1 | 62.3 | 46.3 | 17.9 |
| F18 | 10.2 | 16.4 | 38.7 | 23 | 2.8 | 6.6 |
| F19 | - | 5.1 | 2.2 | 17.5 | - | 2.4 |
| F20 | 1.2 | 12.3 | 7 | 68.4 | 55.4 | 15.3 |
| F21 | 2.5 | 13.6 | 0.3 | 6.9 | - | 4.3 |
| F22 | 6.1 | 7.8 | 2 | 40.4 | - | 3.3 |
| F23 | 0.5 | 12.4 | 3.4 | 25.9 | - | 2.1 |
| F24 | 20.8 | 39.6 | 14.5 | 76.6 | 53.7 | 8.7 |
| F25 | 24.6 | 43.1 | 26 | 82.5 | 52.1 | 0.3 |
| F26 | 3.4 | 15.1 | 11.8 | 20. 3 | 13.9 | - |
| F27 | 17.1 | 28.4 | 30.7 | 75.8 | 54.4 | 8.5 |
| F28 | 10.3 | 14.4 | 22.1 | 37.1 | 14.4 | - |
| F29 | 6.1 | 34.8 | 26.4 | / | / | / |
| F30 | - | 16.7 | 34 | 69.8 | 44 | 2.6 |
| a Metalaxyl | / | / | / | 93.4 | 84.1 | / |
| b Validamycin | / | / | 36.4 | / | / | / |
| c Carbendazim | 89 | 100 | / | / | / | 88.1 |
a Metalaxyl at 10 mg/L; b Validamycin at 100 mg/L; c Carbendazim at 50 mg/L; d “-”means no activity; e “/”means not tested. FO means F. oxysporum f.sp.cubense; CG means C. gloeosporioides (Penz.); RS means R. solani; PL means P. litchi; PN means P. nicotianae; OC means O. citriaurantii ex Persoon.
2.3. Leaf-Piece Assays
The alkaloids and their derivatives compounds F1–30 were evaluated in in vivo fungicidal tests (litchi leaf-piece assays), against the phytopathogenic of P. litchii. The resulting data (Table 2) revealed that several compounds displayed potential fungicidal activity. When the R1 was4-nitrophenyl, these compounds exhibited higher preventative or curative activities than those with phenyl, 4-methoxyphenyl, 3,4,5-trimethoxyphenyl, 4-trifluoromethylphenyl, or 4-chlorophenyl as the R1. For example, compounds F9 and F10 showed higher preventative activity against P. litchi on litchi leaf-piece assays; compound F6 had better curative activity against P. litchi on litchi leaf-piece assays. The types of the substituents on the R2 and R3 had a significant influence on the fungicidal activities. For instance, the ethyl group (F1, F6, F11, F16, F21, and F26) and the isopropyl group (F3, F8, F13, F18, F23, and F28) displayed higher curative activity than preventative, and compound F11 had the highest curative activity against P. litchi on litchi leaf-piece assay. Comparatively, the 2-pyridyl group (F4, F9, F14, F19, F24, and F29) and the 2-chlorophenyl group (F5, F10, F15, F20, F25, and F30) exhibited higher preventative activity, and compound F4 not only showed excellent preventative activity (92.6%) much better than the activity of metalaxyl (59.3%) against P. litchi on litchi leaf-piece assay, but also had a good curative activity (59.3%) against P. litchi. All the alkaloids and their derivatives had no phytotoxicity to the leaf pieces on the assay.

Table 2.
Fungicidal activities of compounds F1–30 against P. litchi by litchi leaf-piece assays (percent inhibition, %; 100 mg/L).
| Compound | Preventative | Curative |
|---|---|---|
| Harmaline | 0 | 44.4 |
| Harmalol | 0 | 7.4 |
| Harmine | 22.2 | 7.4 |
| Harmol | 7.4 | 51.6 |
| F1 | 7.4 | 37 |
| F2 | 14.8 | 29.6 |
| F3 | 14.8 | 29.6 |
| F4 | 92.6 | 59.3 |
| F5 | 44.4 | 44.4 |
| F6 | 0 | 51.9 |
| F7 | 29.6 | 37 |
| F8 | 7.4 | 37 |
| F9 | 66.7 | 29.6 |
| F10 | 51.9 | 0 |
| F11 | 22.2 | 63 |
| F12 | 22.2 | 33.3 |
| F13 | / b | / |
| F14 | 0 | 22.2 |
| F16 | 14.8 | 44.4 |
| F17 | 37 | 0 |
| F18 | 7.4 | 51.9 |
| F19 | 0 | 44.4 |
| F20 | 22.2 | 22.2 |
| F21 | 7.4 | 29.6 |
| F22 | 22.2 | 22.2 |
| F23 | 0 | 29.6 |
| F24 | 7.4 | 29.6 |
| F25 | 22.2 | 14.8 |
| F26 | 0 | 29.6 |
| F27 | 29.6 | 7.4 |
| F28 | 14.8 | 37 |
| F29 | / | / |
| F30 | 22.2 | 14.8 |
| a Metalaxyl | 59.3 | 66.7 |
a Metalaxyl at 10 mg/L; b “/” means not tested.
3. Experimental Section
3.1. General Synthesis
All anhydrous solvents were dried and purified by using standard techniques. The synthetic routes are given in Figure 2 and Scheme 1.
Synthetic Procedure for the Precursors of Target Compounds F1–30
Synthesis of N-ethyl-1-phenyl-9H-pyrido[3,4-b]indole-3-formamide (F1). l-Tryptamine (20.40 g, 0.1 mol) was added in three neck flasks, and 100 mL of acetic acid was added with stirring and dissolved by heating. Press material quality 1:1 ratio dropping weighed benzaldehyde (10.62 g, 0.1 mol), the reaction was heated to reflux, and the reaction progress was followed by thin layer chromatography (TLC). When TLC showed the point of material starting to disappear, we stopped the reaction. The resulting solution was cooled by suction filtration, and washed with water as eluent to remove acetic acid and give1-phenyl-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylic acid [21,26,27,28,29,30] as a white powdery solid (24.62 g, 85.27%).
Four milliliters of SOCl2 were slowly dropped into 40 mL of methanol in the ice salt bath, and then the ice bath was removed after the mixture was stirred for 20 min. Previous product 1-phenyl-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylic acid 11.68 g was added to the reaction mixture, then warmed slowly to reflux and the reaction was refluxed for 4 h, using TLC to track the progress of the reaction. When the reaction was completed, the reaction solution was stirred into 500 mL of ice water after it was cooled, and the pH was adjusted with 30% NaOH to neutral; at this time, the solution appeared as a heavy white precipitate. The precipitate was washed with water and filtered, then dried in a vacuum to give 1-phenyl-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester as a white powdery solid (9.26 g, 65.20%).
The product of the previous step was 1-phenyl-1,2,3,4-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester, of which 6.84 g was added in three neck flasks that were assembled in the ice salt bath; DMF was used as menstruum and KMnO4 was added according to the molar ratio of 1:1.2 ratio, reacting for 0.5 h in the ice bath. The reaction progress was followed by TLC. The mixture was cooled to room temperature after the reaction was finished, and then filtered in a vacuum after stirring for 12 h. The filtrate was added to water and stirred sufficiently to give the precipitated solid, the solution was filtered by suction, then the solid was washed with water to give1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester as a pale yellow solid (3.66 g, 52.71%).
1-Phenyl-9H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester (6.13 g) and ethanol (25.0 mL) were added into a 100 mL flask, then the flask was heated in an oil bath with stirring, and then 1 mL aqueous solution containing 0.5 g of sodium hydroxide was added dropwise in the pot. The, reaction was heated for 2 h and followed by TLC. Dilute hydrochloric acid was added dropwise until pH = 5–6 after completion of the reaction; the mixture was cooled in a refrigerator at 4 °C overnight, then filtered, washed with water, and dried to give 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylic acid as a yellow solid (4.51 g, 65.1%).
1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylic acid (5.76 g) was added to a 50 mL flask that was installed in a reflux condenser tube, drier, and exhaust absorption device, then 20 mL of thionyl chloride was added to the flask with stirring and the mixture was heated at reflux for 1 h. The condensed reflux into recovery device after the reaction was completed, and then the excess thionyl chloride was distilled off to give1-phenyl-9H-pyrido[3,4-b]indole-3-carbonyl chloride as an off-white solid powder (3.74 g, 67.1%).
The solid that was obtained at the previous step was dissolved with dichloromethane, then the solution, under an ice bath with stirring, was added dropwise slowly to a 50-mL single neck flask that had 1.5 g 30% ethylamine solution mixed with 2.0 g of alcohol of triethylamine. The mixture was stirred at room temperature for 30 min, and evaporated to dryness by rotary evaporation of dichloromethane, then dissolved in an appropriate amount of methanol. After that the solution was poured into water and cooled with stirring to sufficiently precipitate solid, and then the mixture was filtered. The resulting residue was washed with the amount of 5% sodium hydroxide solution and water until neutral, and finally dried to give F1 as a yellow solid.
The target compounds F2–30 were prepared by following the same procedure as for compound F1. The corresponding starting materials were all commercially available.
3.2. Spectral Data
N-Ethyl-1-phenyl-9H-pyrido[3,4-b]indole-3-formamide (C20H17N3O, m.w. 315) (F1): yield, 79.54%; m.p. 210–227 °C. IR (KBr) cm−1: 3430 (νN-H), 2924, 2850 (νC-H), 1647 (νC=O), 1023 (νC-N); 1H-NMR (600 MHz, DMSO) δ 11.82 (1H, s, N(9)H, c), 8.82 (1H, s, C(4)H, c), 8.70 (1H, t, J = 6.0 Hz, CO-NH), 8.43–8.39 (1H, m, C(5)H, c), 8.15 (2H, dd, J = 12.3, 5.2 Hz, Ph(2,6)H), 8.03–8.01 (1H, m, C(8)H, c), 7.65 (2H, dd, J = 7.4, 6.3 Hz, C(7)H, c, Ph(4)H), 7.60–7.57 (2H, m, Ph(3,5)H), 7.34–7.31 (1H, m, C(6)H, c), 3.44–3.40 (2H, m, CH2), 1.18 (3H, t, J = 7.2 Hz, CH3).
N,N-Diethyl-1-phenyl-9H-pyrido[3,4-b]indole-3-formamide (C22H21N3O, m.w. 343) (F2): yield, 57.1%; a pale yellow solid. m.p. 210–227 °C. IR (KBr) cm−1: 3449 (νN-H), 2918, 2851 (νC-H), 1623 (νC=O), 1022 (νC-N); 1H-NMR (600 MHz, DMSO) δ 11.71 (1H, s, N(9)H, c), 8.42 (1H, s, C(4)H, c), 8.35 (1H, d, J = 7.8 Hz, C(5)H, c), 8.02 (2H, d, J = 7.4 Hz, Ph(2,6)H), 7.67 (1H, d, J = 8.2 Hz, C(8)H, c), 7.63 (2H, t, J = 7.6 Hz, Ph(3,5)H), 7.56 (2H, m, J = 19.5, 7.5 Hz, C(7)H, c, Ph(4)H), 7.29 (1H, t, J = 7.4 Hz, C(6)H, c), 3.50 (4H, m, J = 13.7, 6.8 Hz, CH2), 1.23–1.18 (6H, m, CH3).
N-Isopropyl-1-phenyl-9H-pyrido[3,4-b]indole-3-formamide (C21H19N3O, m.w. 329) (F3): yield, 47.27%; a pale yellow solid. m.p. 188–191 °C. IR (KBr) cm−1: 3430 (νN-H), 2924, 2850 (νC-H), 1647 (νC=O), 1252 (νC-N); 1H-NMR (600 MHz, DMSO) δ 11.82 (1H, s, N(9)H, c), 8.82 (1H, s, C(4)H, c), 8.41 (1H, d, J = 7.9 Hz, CO-NH), 8.28 (1H, d, J = 8.3 Hz, C(5)H, c), 8.12 (2H, d, J = 7.3 Hz, Ph(2,6)H), 7.67 (3H, dd, J = 17.0, 8.1 Hz, C(8)H, c, Ph(3,5)H), 7.59 (2H, dd, J = 17.0, 7.7 Hz, C(7)H, c, Ph(4)H), 7.35–7.23 (1H, m, C(6)H, c), 4.20 (1H, m, J = 13.4, 6.7 Hz, CH), 1.26 (6H, d, J = 6.6 Hz, CH3).
N-(2-Pyridyl)-1-phenyl-9H-pyrido[3,4-b]indole-3-formamide (C23H16N4O, m.w. 364) (F4): yield, 81.49%; a yellow-brown solid. m.p. 182–183 °C. IR (KBr) cm−1: 3447, 3423 (νN-H), 1623 (νC=O), 1245 (νC-N), 1493 (νC=C); 1H-NMR (600 MHz, DMSO) δ 12.05 (1H, s, N(9)H, c), 10.66 (1H, s, CO-NH), 9.05 (1H, s, C(4)H, c), 8.88 (1H, s, C(5)H, c), 8.49 (1H, d, J = 7.6 Hz, Py(3)H), 8.39 (2H, d, J = 6.5 Hz, Ph(2,6)H), 8.35 (1H, d, J = 7.8 Hz, C(8)H, c), 8.13 (1H, d, J = 5.7 Hz, Py(5)H), 7.72 (1H, s, C(7)H, c), 7.60 (2H, d, J = 7.3 Hz, Ph(3,5)H), 7.35 (2H, d, J = 7.7 Hz, Ph(4)H, C(6)H, c), 7.31 (1H, d, J = 6.8 Hz, Py(4)H), 7.20 (1H, s, Py(6)H).
N-(2-Chlorophenyl)-1-phenyl-9H-pyrido[3,4-b]indole-3-formamide (C24H16ClN3O, m.w. 397) (F5): yield, 56.98%; a brown-yellow solid. m.p. 206–207 °C. IR (KBr) cm−1: 3424 (νN-H), 1657 (νC=O), 1497 (νC=C), 742 (νC-Cl); 1H-NMR (600 MHz, DMSO) δ 12.07 (1H, s, N(9)H, c), 11.06 (1H, s, CO-NH), 9.02 (1H, s, C(4)H, c), 8.63–8.60 (1H, m, C(5)H, c), 8.49 (1H, d, J = 7.8 Hz, Ph2(6)H), 8.19 (2H, d, J = 7.2 Hz, Ph1(2,6)H), 7.73 (1H, d, J = 8.2 Hz, C(8)H, c), 7.70 (2H, t, J = 7.6 Hz, Ph1(3,5)H), 7.67–7.61 (2H, m, Ph2(3)H, C(7)H, c), 7.61–7.60 (1H, m, Ph1(4)H), 7.45 (1H, t, J = 7.7 Hz, Ph2(4)H), 7.36 (1H, t, J = 7.4 Hz, C(6)H, c), 7.20–7.17 (1H, m, Ph2(4)H).
N-Ethyl-1-(4-nitrophenyl)-9H-pyrido[3,4-b]indole-3-formamide (C20H16N4O3, m.w. 360) (F6): yield, 77.82%; a bright yellow solid. m.p. 269–272 °C. IR (KBr) cm−1: 3435 (νN-H), 2921, 2848 (νC-H), 1624 (νC=O), 1249 (νC-N), 1520, 1347 (νNO2); 1H-NMR (600 MHz, DMSO) δ 12.20 (1H, s, N(9)H, c), 9.02 (1H, s, C(4)H, c), 8.48 (2H, d, J = 3.1 Hz, Ph(2,6)H), 8.47–8.46 (2H, m, Ph(3,5)H), 8.46–8.44 (1H, m, C(5)H, c), 8.32 (1H, d, J = 8.7 Hz, CO-NH), 7.72 (1H, t, J = 7.8 Hz, C(8)H, c), 7.63 (1H, dd, J = 11.9, 4.6 Hz, C(7)H, c), 7.37–7.34 (1H, m, C(6)H, c), 2.80 (2H, m, J = 11.9, 6.0 Hz, CH2), 1.14 (3H, t, J = 7.3 Hz, CH3).
N,N-Diethyl-1-(4-nitrophenyl)-9H-pyrido[3,4-b]indole-3-formamide (C22H20N4O3, m.w. 388) (F7): yield, 66.08%; a yellow-brown solid. m.p. 252–254 °C. IR (KBr) cm−1: 3446 (νN-H), 2925, 2851 (νC-H), 1608 (νC=O), 1105 (νC-N), 1515, 1342 (νNO2); 1H-NMR (600 MHz, DMSO) δ 11.97 (1H, s, N(9)H, c), 8.53 (1H, s, C(4), c), 8.46 (2H, d, J = 8.8 Hz, Ph(2,6)H), 8.39 (1H, d, J = 7.9 Hz, C(5)H, c), 8.31 (2H, d, J = 8.6 Hz, Ph(3,5)H), 7.69 (1H, d, J = 8.1 Hz, C(8)H, c), 7.61 (1H, t, J = 7.7 Hz, C(7)H, c), 7.32 (1H, t, J = 7.5 Hz, C(6)H, c), 3.53–3.46 (4H, m, CH2), 1.24–1.19 (6H, m, CH3).
N-Isopropyl-1-(4-nitrophenyl)-9H-pyrido[3,4-b]indole-3-formamide (C21H18N4O3, m.w. 374) (F8): yield, 84.58%; a pale yellow solid. m.p. 260–262 °C. IR (KBr) cm−1: 3426 (νN-H), 2918, 2850 (νC-H), 1652 (νC=O), 1249 (νC-N), 1519, 1345 (νNO2); 1H-NMR (600 MHz, DMSO) δ 12.02 (1H, s, N(9)H, c), 8.91 (1H, s, C(4)H, c), 8.47 (2H, d, J = 4.8 Hz, Ph(2,6)H), 8.43 (2H, d, J = 8.7 Hz, Ph(3,5)H), 8.34 (1H, d, J = 8.4 Hz, C(5)H, c), 8.31 (1H, d, J = 8.6 Hz, CO-NH), 7.69 (1H, d, J = 8.9 Hz, C(8)H, c), 7.66–7.63 (1H, m, C(7)H, c), 7.35 (1H, d, J = 7.2 Hz, C(6)H, c), 4.22 (1H, m, J = 13.5, 6.7 Hz, CH), 1.26 (6H, d, J = 6.6 Hz, CH3).
N-(2-Pyrido)-1-(4-nitrophenyl)-9H-pyrido[3,4-b]indole-3-formamide (C23H15N5O3, m.w. 409) (F9): yield, 62.69%; a yellow-brown solid. m.p. 253–255 °C. IR (KBr) cm−1: 3427 (νN-H), 1625 (νC=O), 1321 (νC-N), 1601, 1495 (νC=C), 1519, 1348 (νNO2); 1H-NMR (600 MHz, DMSO) δ 12.15 (1H, s, N(9)H, c), 10.61 (1H, s, CO-NH), 9.02 (1H, s, C(4)H, c), 8.53 (1H, d, J = 7.0 Hz, C(5)H, c), 8.47 (2H, d, J = 4.2 Hz, Ph(2,6)H), 8.31 (2H, d, J = 8.7 Hz, Ph(3,5)H), 8.17 (1H, d, J = 8.7 Hz, Py(3)H), 7.84 (1H, d, J = 8.7 Hz, C(5)H, c), 7.72 (1H, d, J = 2.7 Hz, Py(5)H), 7.68–7.65 (1H, m, C(7)H, c), 7.61–7.57 (1H, m, C(6)H, c), 7.37 (1H,d, J = 7.5 Hz, Py(4)H), 7.35–7.29 (1H, m, Py(6)H).
N-(2-Chlorophenyl)-1-(4-nitrophenyl)-9H-pyrido[3,4-b]indole-3-formamide (C24H15ClN4O3, m.w. 442) (F10): yield, 43.6%; a yellow-brown solid. m.p. 277–278 °C. IR (KBr) cm−1: 3439 (νN-H), 1629 (νC=O), 1495 (νC=C), 737 (νC-Cl), 1519, 1348 (νNO2); 1H-NMR (600 MHz, DMSO) δ 12.15 (1H, s, N(9)H, c), 10.61 (1H, s, CO-NH), 9.02 (1H, s, C(4)H, c), 8.53 (1H, d, J = 6.8 Hz, C(5)H, c), 8.47 (2H, d, J = 4.2 Hz, Ph1(2,6)H), 8.43 (1H, d, J = 8.7 Hz, Ph2(6)H), 8.39–8.37 (1H, m, Ph2(3)H), 8.31 (2H, d, J = 8.7 Hz, Ph1(3,5)H), 8.17 (1H, d, J = 8.7 Hz, C(8)H, c), 7.84 (1H, d, J = 8.7 Hz, C(7)H, c), 7.72 (1H, d, J = 2.7 Hz, Ph2(5)H), 7.68–7.65 (1H, m, C(6)H, c), 7.37 (1H, d, J = 7.5 Hz, Ph2(4)H).
N-Ethyl-1-(4-methoxyphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C21H19N3O2, m.w. 345) (F11): yield, 41.48%; a pale yellow solid. m.p. 171–173 °C. IR (KBr) cm−1: 3429 (νN-H), 2923, 2853 (νC-H), 1653 (νC=O), 1249 (νC-N), 1249, 1119 (νC-O-C); 1H-NMR (600 MHz, DMSO) δ 11.80 (1H, s, N(9)H, c), 8.76 (1H, s, C(4)H, c), 8.69 (1H, t, J = 6.1 Hz, CO-NH), 8.39 (1H, t, J = 7.2 Hz, C(5)H, c), 8.13 (2H, dd, J = 9.2, 2.4 Hz, Ph(2,6)H), 7.69 (1H, d, J = 8.2 Hz, C(8)H, c), 7.58 (1H, dd, J = 11.7, 4.6 Hz, C(7)H, c), 7.31 (1H, dd, J = 14.3, 7.1 Hz, C(6)H, c), 7.19 (2H, dd, J = 9.8, 6.3 Hz, Ph(3,5)H), 3.89 (3H, s, J = 2.9 Hz, OCH3), 3.45–3.38 (2H, m, CH2), 1.23–1.14 (3H, m, CH3).
N,N-Diethyl-1-(4-methoxyphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C23H23N3O2, m.w. 373) (F12): yield, 36.66%; a pale yellow solid. m.p. 225–226 °C. IR (KBr) cm−1: 3427(νN-H), 2921, 2851 (νC-H), 1609 (νC=O), 1243 (νC-N), 1243, 1113 (νC-O-C); 1H-NMR (600 MHz, DMSO) δ 11.67 (1H, s, N(9)H, c), 8.36 (1H, s, C(4)H, c), 8.35–8.32 (1H, m, C(5)H, c), 7.99 (2H, d, J = 8.7 Hz, Ph(2,6)), 7.67 (1H, d, J = 8.2 Hz, C(8)H, c), 7.57 (1H, t, J = 7.6 Hz, C(7)H, c), 7.28 (1H, t, J = 7.5 Hz, C(6)H, c), 7.19 (2H, d, J = 8.7 Hz, Ph(3,5)H), 3.88 (s, 3H, OCH3), 3.49 (4H, m, J = 13.6, 6.8 Hz, CH2), 1.23–1.18 (6H, m, CH3).
N-Isopropyl-1-(4-methoxyphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C22H21N3O2, m.w. 359) (F13): yield, 23.92%; a yellow solid. m.p. 188–189 °C. IR (KBr) cm−1: 3430 (νN-H), 2924, 2850 (νC-H), 1648 (νC=O), 1176 (νC-N), 1248, 1176 (νC-O-C); 1H-NMR (600 MHz, DMSO) δ 11.78 (1H, s, N(9)H, c), 8.77 (1H, s, C(4)H, c), 8.39 (1H, d, J = 7.9 Hz, C(5)H, c), 8.28 (1H, d, J = 8.3 Hz, CO-NH), 8.09 (2H, dd, J = 6.8, 4.8 Hz, Ph(2,6)H), 7.68 (1H, dd, J = 8.6, 2.1 Hz, C(8)H, c), 7.60−7.58 (1H, m, C(7)H, c), 7.37−7.26 (1H, m, C(6)H, c), 7.22−7.20 (2H, m, Ph(3,5)H), 4.21−4.18 (1H, m, CH), 3.89 (3H, s, J = 0.9 Hz, OCH3), 1.26 (6H, dd, J = 6.6, 1.4 Hz, CH3).
N-(2-Pyridyl)-1-(4-methoxyphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C24H18N4O2, m.w. 394) (F14): yield, 40.36%; a brown solid. m.p. 244–245 °C. IR (KBr) cm−1: 3414, 3340 (νN-H), 1686 (νC=O), 1300 (νC-N), 1608, 1512 (νC=C), 1252, 1176 (νC-O-C); 1H-NMR (600 MHz, DMSO) δ 12.14 (1H, s, N(9)H, c), 10.66 (1H, s, CO-NH), 9.05 (1H, s, C(4)H, c), 8.40 (2H, d, J = 4.9 Hz, Py(3)H, C(5)H, c), 8.09 (2H, t, J = 8.6 Hz, Ph(2,6)H), 7.94–7.91 (2H, m, Py(5)H, C(8), c), 7.73–7.71 (1H, m, C(7)H, c), 7.64–7.62 (1H, m, C(6)H, c), 7.28–7.25 (2H, m, Ph(3,5)H), 6.98 (1H, d, J = 8.8 Hz, Py(4)H), 6.88–6.83 (1H, m, Py(6)H), 3.91 (3H, t, J = 2.8 Hz, OCH3).
N-(2-Chlorophenyl)-1-(4-methoxyphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C25H18ClN3O2, m.w. 427) (F15): yield, 7.66%; a yellow-brown solid. m.p. 250–251 °C. IR (KBr) cm−1: 3422 (νN-H), 1665 (νC=O), 1510 (νC=C), 746 (νC-Cl), 1249, 1114 (νC-O-C); 1H-NMR (600 MHz, DMSO) δ 12.02 (1H, s, N(9)H, c), 11.08 (1H, s, CO-NH), 8.98 (1H, d, J = 13.0 Hz, C(4)H, c), 8.62 (1H, d, J = 8.1 Hz, C(5)H, c), 8.47 (1H, d, J = 7.7 Hz, Ph2(6)H), 8.15 (2H, d, J = 8.8 Hz, Ph1(2,6)H), 7.74–7.71 (1H, m, C(7)H, c), 7.62 (2H, dd, J = 16.7, 8.0 Hz, C(8)H, c, Ph2(3)H), 7.46 (1H, t, J = 7.8 Hz, C(6)H, c), 7.35 (1H, t, J = 7.5 Hz, Ph2(5)H), 7.25 (2H, d, J = 8.6 Hz, Ph1(3,5)H), 7.19 (1H, t, J = 7.7 Hz, Ph2(4)H), 3.91 (3H, s, OCH3).
N-Ethyl-1-(3,4,5-trimethoxyphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C23H23N3O4, m.w. 405) (F16): yield, 54.13%; a pale yellow solid. m.p. 241–243 °C. IR (KBr) cm−1: 3429 (νN-H), 2925, 2850 (νC-H), 1653 (νC=O), 1250 (νC-N), 1250, 1104 (νC-O-C); 1H-NMR (600 MHz, DMSO) δ 11.73 (1H, s, N(9)H, c), 8.92 (1H, d, J = 20.2 Hz, C(4)H, c), 8.41 (1H, t, J = 7.9 Hz, CO-NH), 7.58 (3H, dd, J = 11.5, 8.2 Hz, Ph(2,6)H, C(8)H, c), 7.30 (2H, ddd, J = 20.1, 13.5, 7.1 Hz, C(7)H, c, C(6)H, c), 4.02 (3H, s, Ph1(4)OCH3), 3.93 (6H, s, Ph1(3,5)OCH3), 3.17 (2H,d, J = 5.2 Hz, CH2), 1.15 (3H,d, J = 2.0 Hz, CH3).
N,N-Diethyl-1-(3,4,5-trimethoxyphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C25H27N3O4, m.w. 433) (F17): yield, 14.84%; a pale yellow solid. m.p. 268–270 °C. IR (KBr) cm−1: 3454 (νN-H), 2929, 2850 (νC-H), 1623 (νC=O), 1238 (νC-N), 1238, 1127 (νC-O-C); 1H-NMR (600 MHz, DMSO) δ 11.72 (1H, s, N(9)H, c), 8.90 (1H, s, C(4)H, c), 8.42 (1H, d, J = 7.8 Hz, C(5)H, c), 8.13 (1H, d, J = 8.4 Hz, C(8)H, c), 7.64–7.54 (3H, m, C(7)H, c, Ph(2,6)H), 7.31 (1H, dd, J = 11.4, 4.4 Hz, C(6)H, c), 4.03 (3H, s, Ph1(4)OCH3), 3.93 (6H, s, Ph1(3,5)OCH3), 3.31–3.03 (4H, m, CH2), 1.21 (6H, t, J = 6.5 Hz, CH3).
N-Isopropyl-1-(3,4,5-trimethoxyphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C24H25N3O4, m.w. 419) (F18): yield, 22.55%; a yellow solid. m.p. 311–317 °C. IR (KBr) cm−1: 3433 (νN-H), 2925, 2855 (νC-H), 1651 (νC=O), 1052 (νC-N), 1252, 1116 (νC-O-C); 1H-NMR (600 MHz, DMSO) δ 11.72 (1H, s, N(9)H, c), 9.03–8.72 (1H, m, CO-NH), 8.44–8.41 (1H, m, C(4)H, c), 8.35 (1H, d, J = 7.9 Hz, C(5)H, c), 7.67 (1H, dd, J = 17.8, 8.2 Hz, C(8), c), 7.61–7.56 (1H, m, C(7)H, c), 7.29–7.27 (1H, m, C(6)H, c), 7.23 (2H, s, Ph(2,6)H), 3.93–3.90 (6H, m, Ph1(3,5)OCH3), 3.78 (3H, d, J = 3.0 Hz, Ph1(4)OCH3), 3.50 (1H, s, CH), 1.23 (6H, m, J = 12.5, 7.0 Hz, CH3).
N-(2-Pyridyl)-1-(3,4,5-trimethoxyphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C26H22N4O4, m.w. 454) (F19): yield, 41.63%; a khaki solid. m.p. 279–281 °C. IR (KBr) cm−1: 3433 (νN-H), 1651 (νC=O), 1252 (νC-N), 1623, 1525 (νC=C), 1252, 1113 (νC-O-C); 1H-NMR (600 MHz, DMSO) δ 11.89 (1H, s, N(9)H, c), 10.58 (1H, s, CO-NH), 9.09 (1H, s, C(4)H, c), 8.50–8.48 (1H, m, C(5)H, c), 8.37 (1H, d, J = 8.3 Hz, Py(3)H), .94 (2H, dd, J = 16.5, 8.4 Hz, C(8)H, c, Py(5)H), 7.63 (2H, d, J = 3.1 Hz, C(7)H, c, C(6), c), 7.35 (2H, ddd, J = 9.6, 6.2, 3.9 Hz, Py(4,6)H), 7.22–7.19 (2H, m, Ph(2,6)H), 3.95–3.92 (6H, m, Ph(3,5)OCH3), 3.87 (3H, s, Ph(4)OCH3).
N-(2-Chlorophenyl)-1-(3,4,5-trimethoxyphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C27H22ClN3O4, m.w. 487) (F20): yield, 10.09%; a bright yellow solid. m.p. 275–278 °C. IR (KBr) cm−1: 3429 (νN-H), 1624 (νC=O), 1484 (νC=C), 749 (νC-Cl), 1250, 1109 (νC-O-C); 1H-NMR (600 MHz, DMSO) δ 12.11 (1H, s, N(9)H, c), 11.07 (1H, s, CO-NH), 9.54–9.53 (1H, m, C(4)H, c), 9.06 (1H, s, Ph2(6)H), 9.01 (1H, s, C(5)H, c), 8.51–8.47 (2H, m, C(8), c, Ph2(6)H), 7.64 (2H, s, C(7), c, C(6)H, c), 7.41 (2H, s, Ph1(2,6)H), 7.26 (1H, s, Ph2(5)H), 7.19–7.18 (1H, m, Ph2(4)H), 3.97–3.92 (6H, m, Ph1(3,5)OCH3), 3.80 (3H, s, Ph1(4)OCH3).
N-Ethyl-1-(4-trifluoromethylphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C21H16F3N3O, m.w. 383) (F21): yield,78.1%; a beige solid. m.p. 261–263 °C. IR (KBr) cm−1: 3428 (νN-H), 2926, 2848 (νC-H), 1625 (νC=O), 1250 (νC-N), 1119 (νCF3); 1H-NMR (600 MHz, DMSO) δ 11.95 (1H, s, N(9)H, c), 8.87 (1H, s, CO-NH), 8.76 (1H, t, J = 6.1 Hz, C(4)H, c), 8.40 (2H, dd, J = 35.2, 8.0 Hz, Ph(2,6)H), 7.99 (2H, d, J = 8.2 Hz, Ph(3,5)H), 7.68 (1H, d, J = 8.2 Hz, C(8)H, c), 7.65–7.58 (1H, m, C(7)H, c), 7.43–7.27 (1H, m, C(6)H, c), 3.38 (2H, q, J = 4.3 Hz, CH2), 1.18 (3H, t, J = 7.2 Hz, CH3).
N,N-Diethyl-1-(4-trifluoromethylphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C23H20F3N3O, m.w. 411) (F22): yield, 83.4%; a beige solid. m.p. 238–239 °C. IR (KBr) cm−1: 3435 (νN-H), 2927, 2851 (νC-H), 1615 (νC=O), 1323 (νC-N), 1122 (νCF3); 1H-NMR (600 MHz, DMSO) δ 11.83 (1H, s, N(9)H, c), 8.49 (1H, s, C(4)H, c), 8.38 (1H, d, J = 7.9 Hz, C(5)H, c), 8.22 (2H, d, J = 8.1 Hz, Ph(2,6)H), 7.99 (2H, d, J = 8.2 Hz, Ph(3,5)H), 7.66 (1H, d, J = 8.2 Hz, C(8)H, c), 7.60 (1H, t, J = 7.6 Hz, C(7)H, c), 7.31 (1H, t, J = 7.4 Hz, C(6)H, c), 3.53–3.47 (4H, m, CH2), 1.20 (6H, t, J = 14.3, 7.0 Hz, CH3).
N-Isopropyl-1-(4-trifluoromethylphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C22H18F3N3O, m.w. 397) (F23): yield, 69.3%; a beige solid. m.p. 245–247 °C. IR (KBr) cm−1: 3437 (νN-H), 2925, 2854 (νC-H), 1646 (νC=O), 1325 (νC-N), 1122 (νCF3); 1H-NMR (600 MHz, DMSO) δ 11.95 (1H, s, N(9)H, c), 8.88 (1H, s, C(4)H, c), 8.43 (1H, d, J = 7.9 Hz, C(5)H, c), 8.33 (3H, d, J = 8.0 Hz, Ph(3,5), C(8)H, c), 8.00 (2H, d, J = 8.2 Hz, Ph(3,5)H), 7.68 (1H, d, J = 8.2 Hz, CO-NH), 7.64–7.58 (1H, m, C(7)H, c), 7.33 (1H, dd, J = 11.3, 4.2 Hz, C(6)H, c), 4.21 (1H, qd, J = 13.3, 6.6 Hz, CH), 1.25 (6H, d, J = 6.6 Hz, CH3).
N-(2-Pyridyl)-1-(4-trifluoromethylphenyl)-9H-pyrido[3,4-b]indole-3-formamide (C24H15F3N4O, m.w. 432) (F24): yield, 57.7%; a beige solid. m.p. 269–271 °C. IR (KBr) cm−1: 3438 (νN-H), 1627 (νC=O), 1322 (νC-N), 1524 (νC=C), 1121 (νCF3); 1H-NMR (600 MHz, DMSO) δ 12.15 (1H, s, N(9)H, c), 10.61 (1H, d, J = 13.9 Hz, CO-NH), 9.10 (1H, s, C(4)H, c), 8.51 (1H, d, J = 7.9 Hz, C(5)H, c), 8.38 (2H, ddd, J = 12.1, 6.9, 3.6 Hz, Py(3), C(8)H, c), 8.34 (2H, d, J = 8.3 Hz, Ph(2,6)H), 8.06 (2H, d, J = 8.1 Hz, Ph(3,5)H), 7.70 (2H, dd, J = 16.2, 8.2 Hz, C(7, 8)H, c), 7.62–7.57 (1H, m, C(6)H, c), 7.41–7.35 (1H, m, Py(4)H), 7.26–7.14 (1H, m, Py(6)H).
N-(2-Chlorophenyl)-1-(4-trifluoromethylphenyl)-9H-pyrido[3,4-b]indole-3-formam-ide (C25H15ClF3N3O, m.w. 465) (F25): yield, 95.6%; a beige solid. m.p. 295–297 °C.IR (KBr) cm−1: 3442 (νN-H), 1641 (νC=O), 1539 (νC=C), 744 (νC-Cl), 1116 (νCF3); 1H-NMR (600 MHz, DMSO) δ 12.17 (1H, s, N(9)H, c), 10.96 (1H, s, CO-NH), 9.05 (1H, s, C(4)H, c), 8.56 (1H, dd, J = 8.2, 1.5 Hz, C(5)H, c), 8.49 (1H, d, J = 7.9 Hz, Ph2(6)H), 8.37 (2H, d, J = 8.1 Hz, Ph1(2,6)H), 8.04 (2H, d, J = 8.2 Hz, Ph1(3,5)H), 7.72 (1H, d, J = 8.2 Hz, C(8)H, c), 7.67–7.63 (1H, m, Ph2(3)H), 7.59 (1H, dd, J = 8.0, 1.4 Hz, C(7)H, c), 7.50–7.42 (1H, m, Ph2(5)H), 7.41–7.33 (1H, m, C(6)H, c), 7.18 (1H, td, J = 7.8, 1.5 Hz, Ph2(4)H).
N-Ethyl-1-(4-chlorophenyl)-9H-pyrido[3,4-b]indole-3-formamide (C20H16ClN3O, m.w. 349) (F26): yield, 49.84%; a gray-yellow solid. m.p. 234–236 °C. IR (KBr) cm−1: 3440 (νN-H), 2926, 2855 (νC-H), 1644 (νC=O), 1091 (νC-N), 746 (νC-Cl); 1H-NMR (600 MHz, DMSO) δ 12.06 (1H, s, N(9)H, c), 8.93 (2H, d, J = 10.2 Hz, Ph1(2,6)H), 8.43 (1H, d, J = 7.8 Hz, CO-NH), 8.07–8.05 (2H, m, C(4, 5)H, c), 7.71–7.69 (3H, m, C(8)H, c, Ph1(3,5)H), 7.63–7.60 (1H, m, C(7)H, c), 7.34 (1H, t, J = 7.1 Hz, C(6)H, c), 3.93 (2H, m, CH2), 1.36–1.05 (3H, m, CH3).
N,N-Diethyl-1-(4-chlorophenyl)-9H-pyrido[3,4-b]indole-3-formamide (C22H20ClN3O, m.w. 377) (F27): yield, 25.64%; a pale yellow solid. m.p. 247–248 °C. IR (KBr) cm−1: 3436 (νN-H), 2925, 2855 (νC-H), 1626 (νC=O), 1093 (νC-N), 747 (νC-Cl); 1H-NMR (600 MHz, DMSO) δ 11.88 (1H, s, N(9)H, c), 8.82 (1H, s, C(4)H, c), 8.41 (1H, d, J = 7.9 Hz, C(5)H, c), 8.21–8.19 (2H, m, Ph1(2,6)H), 7.70–7.68 (3H, m, C(8)H, c, Ph1(3,5)H), 7.62–7.59 (1H, m, C(7)H, c), 7.32 (1H, t, J = 7.1 Hz, C(6)H, c), 3.16 (4H, q, J = 5.2 Hz, CH2), 1.16 (6H, m, J = 26.0, 7.2 Hz, CH3).
N-Isopropyl-1-(4-chlorophenyl)-9H-pyrido[3,4-b]indole-3-formamide (C21H18ClN3O, m.w. 363) (F28): yield, 60.15%; a pale yellow solid. m.p. 257–259 °C. IR (KBr) cm−1: 3439 (νN-H), 2969, 2927 (νC-H), 1648 (νC=O), 1092 (νC-N), 746 (νC-Cl); 1H-NMR (600 MHz, DMSO) δ 11.87 (1H, s, N(9)H, c), 8.85 (1H, d, J = 17.0 Hz, C(5)H, c), 8.45–8.28 (2H, m, Ph1(2,6)H), 8.22–8.07 (2H, m, C(4)H, c, CO-NH), 7.70 (3H, ddd, J = 15.4, 8.7, 5.3 Hz, C(8)H, c, Ph1(3,5)H), 7.64–7.56 (1H, m, C(8 )H, c), 7.37–7.28 (1H, m, C(6)H, c), 4.24–4.13 (1H, CH, m), 1.25 (6H, d, J = 6.6 Hz, CH3).
N-(2-Pyridyl)-1-(4-chlorophenyl)-9H-pyrido[3,4-b]indole-3-formamide (C23H15ClN4O, m.w. 398) (F29): yield, 7.29%; a pale yellow solid. m.p. 263–265 °C. IR (KBr) cm−1: 3436 (νN-H), 1628 (νC=O), 1455 (νC=C), 1094 (νC-N), 747 (νC-Cl); 1H-NMR (600 MHz, DMSO) δ 12.03 (1H, s, N(9)H, c), 8.93 (1H, s, N(9)H, c), 8.43 (2H, d, J = 7.9 Hz, Ph1(2,6)H), 8.10 (3H, t, J = 8.5 Hz, C(4,5)H, c, Py(3)H), 7.70 (5H, t, J = 8.5 Hz, C(7,8)H, c, Ph1(3,5)H, Ph2(5)H), 7.62 (1H, t, J = 7.1 Hz, C(6)H, c), 7.32 (2H, dd, J = 28.9, 21.6 Hz, Ph2(4,6)H).
N-(2-Chlorophenyl)-1-(4-chlorophenyl)-9H-pyrido[3,4-b]indole-3-formamide (C24H15Cl2N3O, m.w. 432) (F30): yield, 35.84%; a pale yellow solid. m.p. 271–273 °C. IR (KBr) cm−1: 3449 (νN-H), 1649 (νC=O), 1594, 1497 (νC=C), 1094 (νC-N), 741 (νC-Cl); 1H-NMR (600 MHz, DMSO) δ 12.16 (1H, s, N(9)H, c), 11.00 (1H, s, CO-NH), 9.02 (1H s, C(4)H, c), 8.58 (1H,t, J = 8.2, 4.1 Hz, C(5)H, c), 8.49 (1H, d, J = 7.9 Hz, Ph2(6)H), 8.24–8.17 (2H, m, Ph1(3,5)H), 7.78–7.72 (3H, m, Ph1(3,5)H, Ph2(3)H), 7.64 (1H, ddd, J = 8.2, 5.8, 1.1 Hz, C(8)H, c), 7.60 (1H, dd, J = 8.0, 1.4 Hz, C(7)H, c), 7.47–7.43 (1H, m, Ph2(5)H), 7.40–7.33 (1H, m, C(6)H, c), 7.22–7.16 (1H, m, Ph2(4)H).
3.3. Fungicidal Activities
The compounds were screened on a broader range of fungal pathogens in mycelial growth tests in artificial media against Fusarium oxysporum f.sp.cubense, Colletotrichum gloeosporioides (Penz.), R. solani, Peronophthora litchii, Phytophthora nicotianae, and O. citriaurantii ex Persoon, at the concentration of 100 μg/mL. Each assay contained three replicates for each concentration. The plates were stored in controlled environment cabinets for between two and seven days, depending on the assay, after which mycelia growth was assessed. Treatments were scored as percentage inhibition of mycelial growth relative to untreated controls. The biological data presented in Table 1 are the mean scores for each treatment across replicates.
3.4. Leaf-Piece Assays
The compounds were also evaluated in leaf-piece assays, at the concentration of 100 μg/mL for P. litchi on young leaves of litchi. The formulated chemicals were applied to leaf pieces prior to inoculation with spores of the pathogen, and also after they had been inoculated with pathogen spores. Each assay contained three replicates for each concentration. Assessments were made three days after inoculation, depending on the assay, after which disease inhibition was assessed. Treatments were scored as percentage inhibition of disease development relative to untreated controls. The biological data presented in Table 2 are the mean scores for each treatment across replicates.
4. Conclusions
In summary, a series of β-carbolines derivatives were designed, synthesized, and first assayed for their fungicidal activities in vitro and in vivo. The results showed that most of these compounds exhibited good fungicidal activities, and these compounds had certain selectivity toward fungi species. Of all the new compounds synthesized, F4 (N-(2-pyridyl)-1-phenyl-9H-pyrido[3,4-b]indole-3-formamide) showed activity almost at the same level as the control compound metalaxyl against P. litchii and P. nicotianae in vitro. Very interestingly, this compound exhibited better activity than the control compound validamycin against R. solani in vitro, and also had excellent preventative activity against P. litchi in vivo. Further studies on the activity mechanism of these compounds are underway in our laboratory.
Acknowledgments
The researchers gratefully acknowledge the grants from the China National Nature Science Foundation (31171870); the China National Nature Science Foundation (31171882) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (20134404110019).
Author Contributions
The listed authors contributed to this work as described in the following. Z.L. was the leader of this experiment and prepared the manuscript. S.C. modified the article. S.Z. and J.L. collected the test samples. Y.Z. synthesized compounds, and Q.W. gave the concepts of work and modified the article. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cao, R.H.; Peng, W.L.; Wang, Z.H. β-Carboline Alkaloids: Biochemical and Pharmacological Functions. Curr. Med. Chem. 2007, 14, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, R.A.; Spencer, I.D. The carbolines. Adv. Heterocycl. Chem. 1964, 3, 79–207. [Google Scholar] [PubMed]
- Allen, J.R.F.; Holmstedt, B.R. The Simple β-Carboline Alkaloids. Phytochemistry 1980, 19, 1573–1582. [Google Scholar] [CrossRef]
- De Meester, C. Genotoxic potential of β-carbolines: A review. Mutat. Res. 1995, 339, 139–153. [Google Scholar] [CrossRef]
- Jahaniani, F.; Ebrahimi, S.A.; Rahbar-Roshandel, N.; Mahmoudian, M. Xanthomicrol is the main cytotoxic component of Dracocephalum kotschyii and a potential anti-cancer agent. Phytochemistry 2005, 66, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nagao, M.; Sugimura, T. Interactions of norharman and harman with DNA. Nucleic Acids. Res. 1977, 4, 3679–3686. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, A.; Ebrahimi, S.; Mahmoudian, M. An in vitro evaluation of human DNA topoisomerase I inhibition by Peganum harmala L. seeds extract and its beta-carboline alkaloids. J. Pharm. Pharm. Sci. 2002, 5, 19–23. [Google Scholar] [PubMed]
- Yamashita, K.; Ohgaki, H.; Wakabayashi, K.; Nagao, M.; Sugimura, T. DNA adducts formed by the comutagens harman and norharman in various tissues of mice. Cancer Lett. 1988, 42, 179–183. [Google Scholar] [CrossRef]
- Funayama, Y.; Nishio, K.; Wakabayashi, K.; Nagao, M.; Shimoi, K.; Ohira, T.; Hasegawa, S.; Saijo, N. Effects of beta- and gamma-carboline derivatives of DNA topoisomerase activities. Mutat. Res. 1996, 349, 183–191. [Google Scholar] [CrossRef]
- Herraiz, T.; Chaparro, C. Human monoamine oxidase enzyme inhibition by coffee and beta-carbolines norharman and harman isolated from coffee. Life Sci. 2006, 78, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Chaparro, C. Human monoamine oxidase is inhibited by tobacco smoke: Beta-carboline alkaloids act as potent and reversible inhibitors. Biochem. Biophys. Res. Commun. 2005, 326, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Kokuba, H. Harmol induces autophagy and subsequent apoptosis in U251MG human glioma cells through the downregulation of survivin. Oncol. Rep. 2013, 29, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Yamada, H.; Moriya, S.; Miyazawa, K. The beta-Carboline Alkaloid Harmol Induces Cell Death via Autophagy but Not Apoptosis in Human Non-small Cell Lung Cancer A549 Cells. Biol. Pharm. Bull. 2011, 34, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- El Gendy, M.A.M.; Soshilov, A.A.; Denison, M.S.; El-Kadi, A.O.S. Transcriptional and posttranslational inhibition of dioxin-mediated induction of CYP1A1 by harmine and harmol. Toxicol. Lett. 2012, 208, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Kawabata, H.; Tomita, I. Enhancing effect of heterocyclic amines and beta-carbolines on UV or chemically induced mutagenesis in E. coli. Mutat. Res. 1992, 268, 287–95. [Google Scholar] [CrossRef]
- Di Giorgio, C.; Delmas, F.; Ollivier, E.; Elias, R.; Balansard, G.; Timon-David, P. In vitro activity of the beta-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmania infantum. Exp. Parasitol. 2004, 106, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Liu, Y.X.; Liu, Y.; Wang, L.Z.; Wang, Q.M. Synthesis and Antiviral and Fungicidal Activity Evaluation of β-Carboline, Dihydro-β-carboline, Tetrahydro-β-carboline Alkaloids, and Their Derivatives. J. Agric. Food Chem. 2014, 62, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.H.; Kim, Y.H.; Park, G.T.; Lee, S.J. Beta-carboline alkaloids harmaline and harmalol induce melanogenesis through p38 mitogen-activated protein kinase in B16F10 mouse melanoma cells. J. Biochem. Mol. Biol. Ens. 2010, 43, 824–829. [Google Scholar] [CrossRef] [PubMed]
- El Gendy, M.A.M.; Soshilov, A.A.; Denisonb, M.S.; El-Kadi, A.O.S. Harmaline and harmalol inhibit the carcinogen-activating enzyme CYP1A1 via transcriptional and posttranslational mechanisms. Food Chem. Toxicol. 2012, 50, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, Y.; Liu, Y.; Huang, Y.; Li, Y.; Wang, Q. Design, synthesis, anti-TMV, fungicidal, and insecticidal activity evaluation of 1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid derivatives based on virus inhibitors of plant sources. Bioorg. Med. Chem. Lett. 2014, 24, 5228–5233. [Google Scholar] [CrossRef] [PubMed]
- Love, B.E. Synthesis of β-carbolines. A review. Org. Prep. Proced. Int. 1996, 28, 1–64. [Google Scholar] [CrossRef]
- Silverman, B.D.; Daniel, E.P.; Mike, P.; Isidore, R. Comparative molecular moment analysis (CoMMA). Perspect. Drug Discov. Des. 1998, 12, 183–196. [Google Scholar] [CrossRef]
- Guan, H.J.; Chen, H.S.; Peng, W.L.; Ma, Y.; Cao, R.H.; Liu, X.D.; Xu, A.L. Design of β-carboline derivatives as DNA-targeting antitumor agents. Eur. J. Med. Chem. 2006, 41, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.H.; Guan, X.D.; Shi, B.X.; Chen, Z.Y.; Ren, Z.H.; Peng, W.L.; Song, H.C. Design, synthesis and 3D-QSAR of β-carboline derivatives as potent antitumor agents. Eur. J. Med. Chem. 2010, 45, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Milen, M.; Hazai, L.; Kolonits, P.; Kalaus, G.; Szabo, L.; GoMoRy, A.; SzaNtay, C. Studies on stereoselective approaches to β-carboline derivatives. Cent. Eur. J. Chem. 2005, 3, 118–136. [Google Scholar] [CrossRef]
- Dai, W.M.; Zhu, H.J.; Hao, X.J. Chiral ligands Dderived from abrine-3-Asymmetric pictet-spengler reaction of abrine methyl ester and synthesis of chiral 1,2,3,4-tetrahydro-β-carbolines as promoters in addition of diethylzinc toward aromatic aldehydes. Tetrahedron Lett. 1996, 37, 5971–5974. [Google Scholar] [CrossRef]
- Kuo, F.M.; Tseng, M.C.; Yen, Y.H.; Chu, Y.H. Microwave accelerated Pictet-Spengler reactions of tryptophan with ketones directed toward the preparation of 1,1-disubstituted indole alkaloids. Tetrahedron 2004, 60, 12075–12084. [Google Scholar] [CrossRef]
- Snyder, H.R.; Hansch, C.H.; Katz, L.; Parmerter, S.M.; Spaeth, E.C. The Synthesis of Derivatives of β-Carboline. II. Syntheses from dl-Tryptophan and Aldehydes. J. Am. Chem. Soc. 1948, 70, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Lippke, K.P.; Schunack, W.G.; Wenning, W. β-carbolines as benzodiazepine receptor ligands, synthesis and benzodiazepine receptor interaction of esters of β-carbolines-3-carboxylic acid. Med. Chem. 1983, 26, 499–503. [Google Scholar] [CrossRef]
- Borisovich, S.B.; Aleksandrovich, N.K.; Vadimovich, K.V. Method of production of 1- and 1,1-disubstituted-4-phenyl-2,3,4,9-tetrahydro-1H-β-carboline. R.U. Patent 2332418, 27 August 2004. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
