Prazosin-Conjugated Matrices Based on Biodegradable Polymers and α-Amino Acids—Synthesis, Characterization, and in Vitro Release Study
Abstract
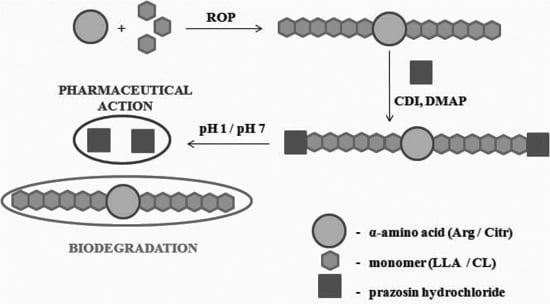
:1. Introduction
2. Results and Discussion
| Sample | Microtox 15 min-EC50 a | Microtox 30 min-EC50 a | Spirotox 24 h-EC50 a |
|---|---|---|---|
| PCL/Arg | 0 | 0 | 0 |
| PLLA/Arg | 0 | 0 | 0 |
| PCL/Citr | 0 | 0 | 0 |
| PLLA/Citr | 0 | 0 | 0 |
| Extract Concentration | 8.33% | 16.66% | 33.33% | 66.66% |
| IR | 0.94 ± 0.06 | 1.11 ± 0.10 | 1.41 ± 0.03 | 3.03 ± 0.30 |




Prazosin Release from the Synthesized Macromolecular Conjugates





| Time (days) | PCL/Arg | PCL/Citr | PLLA/Arg | PLLA/Citr | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WL (%) | ηinh a | Δ ηinh (%) | WL (%) | ηinha | Δ ηinh (%) | WL (%) | ηinha | Δ ηinh (%) | WL (%) | ηinha | Δ ηinh (%) | |
| 7 | 3.3±0.8 | 0.10 | 9 | 4.5 ± 0.4 | 0.07 | 13 | 3.2 ± 0.2 | 0.27 | 3 | 5.1 ± 0.2 | 0.28 | 6 |
| 14 | 7.5 ± 0.4 | 0.10 | 9 | 8.6 ± 0.5 | 0.07 | 13 | 6.6 ± 0.6 | 0.26 | 6 | 7.3 ± 0.3 | 0.27 | 9 |
| 21 | 11.8 ± 0.8 | 0.09 | 18 | 15.8 ± 0.9 | 0.06 | 25 | 9.8 ± 0.9 | 0.24 | 12 | 13.3 ± 0.9 | 0.25 | 14 |
| 28 | 16.4 ± 0.6 | 0.09 | 18 | 26.1 ± 0.2 | 0.06 | 25 | 14.9 ± 0.8 | 0.23 | 15 | 18.2 ± 0.6 | 0.23 | 20 |

3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Macromolecular Conjugates of Prazosin
3.2.2. Synthesis of Prazosin-Conjugated PCL/Arg and PCL/Citr
3.2.3. Synthesis of Prazosin-Conjugated PLLA/Arg and PLLA/Citr
3.2.4. Toxicity Assays
3.2.5. The Umu-Test
3.2.6. In Vitro Prazosin Release Studies
3.2.7. Hydrolytic Degradation
3.3. Characterization Techniques
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bullock, J.; Boyle, J.; Wang, M.B. Physiology (NMS); Elsevier Urban & Partner: Wroclaw, Poland, 2004; pp. 28–75. [Google Scholar]
- Janiec, W. Pharmacodynamics; PZWL: Warsaw, Poland, 2008; pp. 5–45. [Google Scholar]
- Brunton, L.; Blumenthal, D.; Buxton, I.; Parker, K. Goodman and Gilman’s. The Pharmacological Basis of Therapeutics; McGraw-Hill: New York, NY, USA, 2007; pp. 33–49. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; PWN: Warsaw, Poland, 2009; pp. 69–87. [Google Scholar]
- Gad, M.Z. Anti-aging effects of l-arginine. J. Adv. Res. 2010, 1, 169–177. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R. Pharmacokinetic and pharmacodynamics properties of oral l-acitrulline and l-arginine: Impact an nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hayase, M.; Hibi, K.; Hosokawa, H.; Yokoya, K.; Fitzgerald, P.J.; Yock, P.G.; Cooke, J.P.; Suzuki, T.; Yeung, A.C. Effect of local drug delivery of l-arginine on in-stent restenosis in humans. Am. J. Cardiol. 2002, 89, 363–367. [Google Scholar] [CrossRef]
- Tousoulis, D.; Antoniades, C.; Tentolouris, C.; Goumas, G.; Stefanadis, C.; Toutouzas, P. Vascular l-Arginine in cardiovascular disease: Dream or reality? Vasc. Med. 2002, 7, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Martina, V.; Masha, A.; Giglardi, V.R.; Brocato, L.; Manzato, E.; Berchio, A.; Massarenti, P.; Settanni, F.; Casa, L.D.; Bergamini, S.; et al. Long-term N-acetylcysteine and l-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care 2008, 31, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, C.M.; Ray, R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 2001, 55, 141–150. [Google Scholar] [CrossRef]
- Li, S.; Liu, L.; Garreau, H.; Vert, M. Lipase-catalyzed biodegradation of poly(ε-caprolactone) blended with various polylactide-based polymers. Biomacromolecules 2003, 4, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Bansal, K.; Kausshik, R.; Kumria, R.; Trehan, A. Poly-ε-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, A.A.; Claes, L.E. In vitro biocompatibility of bioresorbable polymers: Poly(l,dl-lactide) and poly(l-lactide-co-glycolide). Biomaterials 1996, 17, 831–839. [Google Scholar] [CrossRef]
- Chamberlain, B.M.; Jazdzewski, B.A.; Pink, M.; Hillmyer, M.A.; Tolman, W.B. Controlled polymerization of dl-lactide and ε-caprolactone by structurally well-defined alkoxo-bridged di- and triyttrium(III) complexes. Macromolecules 2000, 33, 3970–3977. [Google Scholar] [CrossRef]
- Li, X.; Adamn, N.W.; Bennett, D.B.; Feij, J.; Kims, S.W. Synthesis of poly(hydroxypropylglutamine-prazosin carbamate) and release studies. Pharm. Res. 1991, 8, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.V.; Sreedhar, V.; Mutalik, S.; Setty, C.M.; Sa, B. Interpenetrating network hydrogel membranes of sodium alginate and poly(vinyl alcohol) for controlled release of prazosin hydrochloride through skin. Int. J. Biol. Macromol. 2010, 47, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Hosny, E.A.; Abbdel Hady, S.S.; Niazy, E.M. Effect of film composition and various penetration enhancers concentration on prazosin release from acrylic polymeric films. Pharm. Acta Helv. 1998, 72, 247–254. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, T. The effect of dendrimers on the pharmacodynamic and pharmacokinetic behaviors of non-covalently or covalently attached drugs. Eur. J. Med. Chem. 2008, 43, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Debek, C.; Oledzka, E.; Nalecz-Jawecki, G.; Kolodziejski, W.L.; Rajkiewicz, M. Segmented polyurethane elastomers derived from aliphatic polycarbonate and poly(ester-carbonate) soft segments for biomedical applications. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3904–3913. [Google Scholar] [CrossRef]
- Kurtoglu, Y.; Mishra, M.K.; Kannan, S.; Kannan, R.M. Drug release characteristics of PAMAM dendrimer-drug conjugates with different linkers. Int. J. Pharm. 2010, 384, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Oledzka, E.; Sobczak, M.; Nalecz-Jawecki, G.; Skrzypczak, A.; Kolodziejski, W. Ampicillin-ester bonded branched polymers: Characterization, cyto-, genotoxicity and controlled drug-release behavior. Molecules 2014, 19, 7543–7556. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Kamysz, W.; Tyszkiewicz, W.; Debek, C.; Kozłowski, R.; Oledzka, E.; Piotrowska, U.; Nalecz-Jawecki, G.; Plichta, A.; Grzywacz, D.; et al. Biodegradable macromolecular conjugates of citropin: Synthesis, characterization and in vitro efficiency study. React. Funct. Polym. 2014, 83, 54–61. [Google Scholar] [CrossRef]
- Davaran, S.; Rashidi, M.R.; Hanaee, J.; Khani, A.; Mahkam, M.; Hashemi, M. Synthesis and degradation characteristics of polyurethanes containing azo derivatives of 5-aminosalicylic acid. J. Bioact. Compat. Polym. 2006, 21, 315–326. [Google Scholar] [CrossRef]
- Oledzka, E.; Sobczak, M.; Kolakowski, M.; Kraska, B.; Kamysz, W.; Kolodziejski, W. Development of creatine and arginine-6-oligomer for the ring-opening polymerization of cyclic esters. Macromol. Res. 2013, 21, 161–168. [Google Scholar] [CrossRef]
- Kumar, L.; Popat, D.; Bansal, A.K. Investigation of the atypical glass transition and recrystallization behavior of amorphous prazosin salts. Pharmaceutics 2011, 3, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Oledzka, E.; Sokolowski, K.; Sobczak, M.; Kolodziejski, W. α-amino acids as initiators of ε-caprolactone and l,l-lactide polymerization. Polym. Int. 2011, 60, 787–779. [Google Scholar] [CrossRef]
- Nalecz-Jawecki, G. Spirotox test-Spirostomum ambiguum acute toxicity test. In Small-scale Freshwater Toxicity Investigations; Blaise, C., Férard, J.F., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 299–322. [Google Scholar]
- International Standard ISO/FDIS 13829. Water Quality-Determination of the Genotoxicity of Water and Waste Water Using the Umu-Test; ISO: Geneva, Switzerland, 2000. [Google Scholar]
- European Pharmacopoeia Commission. European Pharmacopoeia; European Directorate for the Quality of Medicines & HealthCare (EDQM): Strasbourg, France, 2014. [Google Scholar]
- Sample Availability: Samples of the conjugates are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oledzka, E.; Sawicka, A.; Sobczak, M.; Nalecz-Jawecki, G.; Skrzypczak, A.; Kolodziejski, W. Prazosin-Conjugated Matrices Based on Biodegradable Polymers and α-Amino Acids—Synthesis, Characterization, and in Vitro Release Study. Molecules 2015, 20, 14533-14551. https://doi.org/10.3390/molecules200814533
Oledzka E, Sawicka A, Sobczak M, Nalecz-Jawecki G, Skrzypczak A, Kolodziejski W. Prazosin-Conjugated Matrices Based on Biodegradable Polymers and α-Amino Acids—Synthesis, Characterization, and in Vitro Release Study. Molecules. 2015; 20(8):14533-14551. https://doi.org/10.3390/molecules200814533
Chicago/Turabian StyleOledzka, Ewa, Anna Sawicka, Marcin Sobczak, Grzegorz Nalecz-Jawecki, Agata Skrzypczak, and Waclaw Kolodziejski. 2015. "Prazosin-Conjugated Matrices Based on Biodegradable Polymers and α-Amino Acids—Synthesis, Characterization, and in Vitro Release Study" Molecules 20, no. 8: 14533-14551. https://doi.org/10.3390/molecules200814533
APA StyleOledzka, E., Sawicka, A., Sobczak, M., Nalecz-Jawecki, G., Skrzypczak, A., & Kolodziejski, W. (2015). Prazosin-Conjugated Matrices Based on Biodegradable Polymers and α-Amino Acids—Synthesis, Characterization, and in Vitro Release Study. Molecules, 20(8), 14533-14551. https://doi.org/10.3390/molecules200814533