Effect of Brewing Duration on the Antioxidant and Hepatoprotective Abilities of Tea Phenolic and Alkaloid Compounds in a t-BHP Oxidative Stress-Induced Rat Hepatocyte Model
Abstract
:1. Introduction
2. Results
2.1. Impact of Brewing Duration on Tea Phenolic Compounds and Caffeine Composition
| Sample 1 | Mean of Dry Extract 2 (g) | Yield (%) | Total Polyphenols 2–3 (g/100g Dry Extract) |
|---|---|---|---|
| ATE-5 | 4.00 ± 0.15 | 20.02 | 20.9 ± 0.3 a |
| ATE-15 | 3.98 ± 0.10 | 19.88 | 20.2 ± 0.1 a |
| ATE-30 | 3.85 ± 0.26 | 19.23 | 18.9 ± 0.3 b |
| Retention Time (min) | Molecular Mass (Da) | [M − H]−/[M + H]+ | Fragment Ions in Negative Mode | Compound Structures 1 | Compound Content (g/100 g of Dry Extract) 1–3 | ||
|---|---|---|---|---|---|---|---|
| ATE-5 | ATE-15 | ATE-30 | |||||
| 1.1 | 170 | 169/- | 125 | GA | 1.43 ± 0.01 a | 1.34 ± 0.03 b | 1.32 ± 0.02 b |
| 3.0 | 306 | 305/- | 179, 125 | EGC | 0.20 ± 0.01 a | 0.19 ± 0.02 a | 0.13 ± 0.01 b |
| 3.5 | 290 | -/291 | - | C | <LQ | <LQ | <LQ |
| 3.7 | 194 | -/195 | 138 | CAF | 6.87 ± 0.02 a | 6.56 ± 0.05 b | 5.87 ± 0.03 c |
| 4.4 | 290 | -/291 | - | EC | 0.42 ± 0.04 a | 0.35 ± 0.02 a,b | 0.32 ± 0.04 b,c |
| 4.5 | 458 | 457/- | 305, 169 | EGCG | 1.10 ± 0.20 a | 0.88 ± 0.03 a | 0.40 ± 0.10 b |
| 5.9 | 442 | 441/- | 289 | ECG | 0.33 ± 0.03 a | 0.29 ± 0.02 a | 0.15 ± 0.04 b |
| Total catechins | 2.05 ± 0.28 a | 1.71 ± 0.09 a | 1.0 ± 0.2 b | ||||
| Total | 10.3 ± 0.3 a | 9.6 ± 0.1 b | 8.2 ± 0.2 c | ||||

2.2. Antioxidant Activity of ATE Compounds

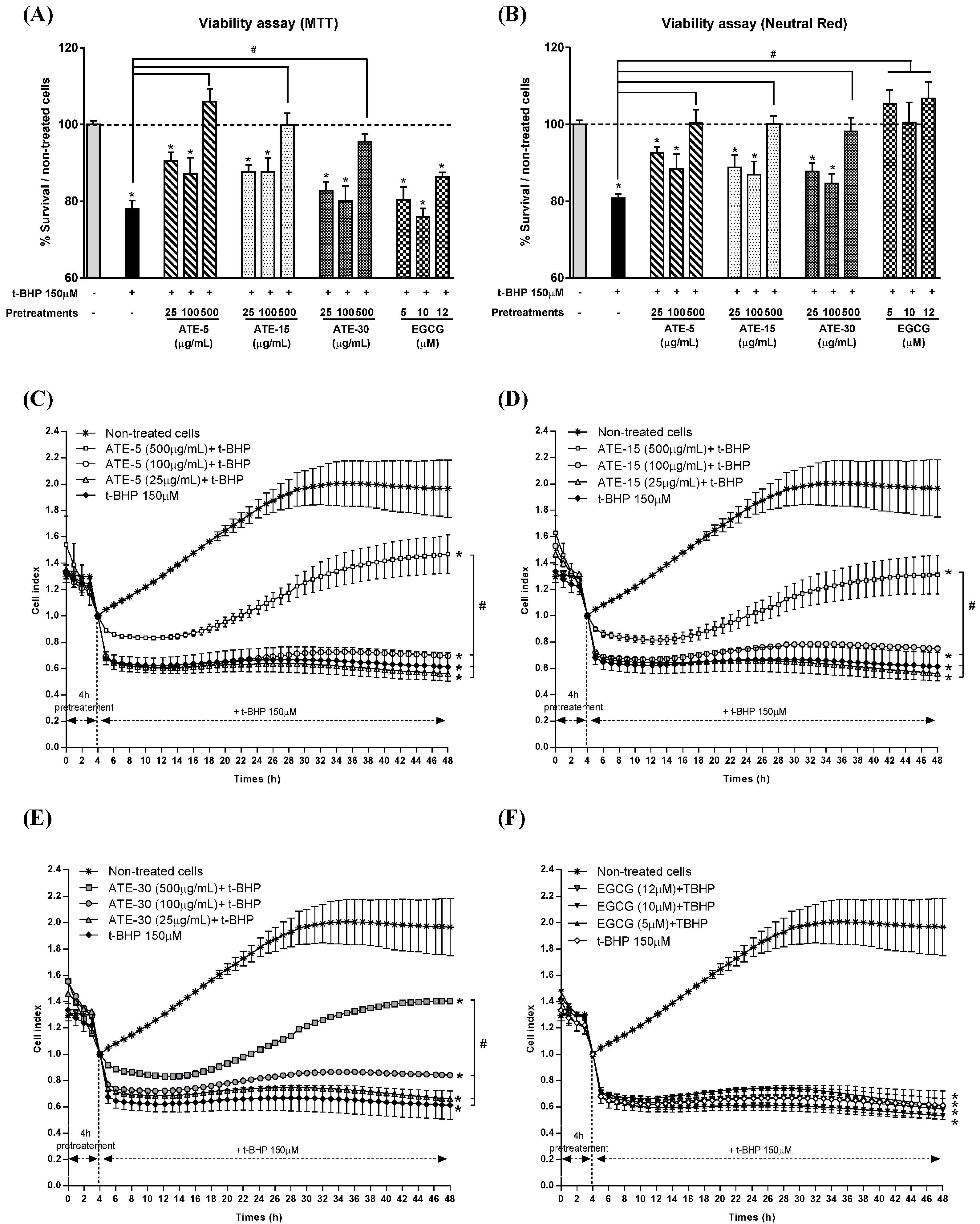
2.3. Effect of ATE or EGCG on Viability of Rat Hepatocytes
2.4. Effect of ATE or EGCG on Mitochondrial Superoxide Anion (O2−) Production and on Mitochondrial Functionality

3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Tea Origin and Aqueous Extract Preparation
4.3. Characterization and Quantification of the Tea Extracts Compounds
4.4. Antioxidant Capacity of ATE Compounds
4.5. Isolation, Cultivation of Rat Hepatocytes and Treatments
4.6. Hepatocyte Viability Tests
4.7. Measurement of Mitochondrial Superoxide Anion (O2−) and of Relative Mitochondrial Activity
4.8. Statistical Analysis
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Roberts, E.A.H. The phenolic substances of manufactured tea. II.—Their origin as enzymic oxidation products in fermentation. J. Sci. Food Agric. 1958, 9, 212–216. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Bhagwat, S.; Haytowitz, D.; Holden, J.; Eldridge, A.L.; Beecher, G.; Aladesanmi, J. Major flavonoids in dry tea. J. Food Compos. Anal. 2005, 18, 487–501. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, H.; Deng, Y. Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 2002, 57, 307–316. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, C.F.; Shen, S.M.; Wang, G.L.; Liu, P.; Liu, Z.M.; Wang, Y.Y.; Du., S.S.; Liu, Z.L.; Deng, Z.W. Antioxidant Phenolic Compounds from Pu-erh Tea. Molecules 2012, 17, 14037–14045. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Tsai, Y.J.; Tsay, J.S.; Lin, J.K. Factors Affecting the Levels of Tea Polyphenols and Caffeine in Tea Leaves. J. Agric. Food Chem. 2003, 51, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Sharangi, A.B. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—A review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Jacob, R.A.; Burri, B.J. Oxidative damage and defense. Am. J. Clin. Nutr. 1996, 63, 985S–990S. [Google Scholar] [PubMed]
- Jaeschke, H.; McGill, M.R.; Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012, 44, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Antioxidant capacity: Which capacity and how to assess it? J. Berry Res. 2011, 1, 169–176. [Google Scholar]
- Takashima, M.; Horie, M.; Shichiri, M.; Hagihara, Y.; Yoshida, Y.; Niki, E. Assessment of antioxidant capacity for scavenging free radicals in vitro: A rational basis and practical application. Free Radic. Biol. Med. 2012, 52, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Astill, C.; Birch, M.R.; Dacombe, C.; Humphrey, P.G.; Martin, P.T. Factors Affecting the Caffeine and Polyphenol Contents of Black and Green Tea Infusions. J. Agric. Food Chem. 2001, 49, 5340–5347. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Golding, J.B.; Nguyen, M.; Roach, P.D. Extraction and isolation of catechins from tea. J. Sep. Sci. 2010, 33, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions, Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, C.; Yang, B.; Kusu, F.; Kotani, A. DPPH radical scavenging activities of 31 flavonoids and phenolic acids and 10 extracts of Chinese materia medica. China J. Chin. Mater. Med. 2009, 34, 1695–1700. [Google Scholar]
- Honzel, D.; Carter, S.G.; Redman, K.A.; Schauss, A.G.; Endres, J.R.; Jensen, G.S. Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J. Agric. Food Chem. 2008, 56, 8319–8325. [Google Scholar] [CrossRef] [PubMed]
- Kučera, O.; Endlicher, R.; Roušar, T.; Lotková, H.; Garnol, T.; Drahota, Z.; Červinková, Z. The effect of tert-butyl hydroperoxide-induced oxidative stress on lean and steatotic rat hepatocytes in vitro. Oxid. Med. Cell. Longev. 2014, 2014, 752506. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, X.; Zhao, X. Powerful protective effects of gallic acid and tea polyphenols on human hepatocytes injury induced by hydrogen peroxide or carbon tetrachloride in vitro. J. Med. Plants Res. 2010, 4, 247–254. [Google Scholar]
- Zhang, Y.; Yang, N.D.; Zhou, F.; Shen, T.; Duan, T.; Zhou, J.; Shi, Y.; Zhu, X.Q.; Shen, H.M. (−)-Epigallocatechin-3-Gallate Induces Non-Apoptotic Cell Death in Human Cancer Cells via ROS-Mediated Lysosomal Membrane Permeabilization. PLoS ONE 2012, 7, e46749. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Igoudjil, A.; Pessayre, D.; Fromenty, B. Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion 2006, 6, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, S.W. Mitochondrial dysfunction in cardiovascular disease. Free Radic. Biol. Med. 2005, 38, 1278–1295. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzàlez-Paramàs, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. Polyphenol-Rich Strawberry Extract Protects Human Dermal Fibroblasts against Hydrogen Peroxide Oxidative Damage and Improves Mitochondrial Functionality. Molecules 2014, 19, 197798–197816. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Quiles, J.L.; Bompadre, S.; Mezzetti, B.; et al. An anthocyanin-rich strawberry extract protects against oxidative stress damage and improves mitochondrial functionality in human dermal fibroblasts exposed to an oxidizing agent. Food Funct. 2014, 5, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Hepatoprotective properties of kombucha tea against TBHP-induced oxidative stress via suppression of mitochondria dependent apoptosis. Pathophysiology 2011, 18, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, J.M.; Cederbaum, A.I. Green tea polyphenol epigallocatechin-3-gallate protects HepG2 cells against CYP2E1-dependent toxicity. Free Radic. Biol. Med. 2004, 36, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kucera, O.; Mezera, V.; Moravcova, A.; Endlicher, R.; Lotkova, H.; Drahota, Z.; Cervinkova, Z. In vitro toxicity of epigallocatechin gallate in rat liver mitochondria and hepatocytes. Oxid. Med. Cell Longev. 2015, 2015, 476180. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Chandel, N.S.; Williamson, E.K.; Schumacker, P.T.; Thompson, C.B. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 1997, 91, 627–637. [Google Scholar] [CrossRef]
- Gottlieb, E.; Armour, S.M.; Harris, M.H.; Thompson, C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome C release during apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.; Bendall, D.S. Production and HPLC analysis of black tea theaflavins and thearubigins during in vitro oxidation. Phytochemistry 1983, 22, 883–887. [Google Scholar] [CrossRef]
- Leung, L.K.; Su, Y.; Chen, R.; Zhang, Z.; Huang, Y.; Chen, Z.Y. Theaflavins in Black Tea and Catechins in Green Tea Are Equally Effective Antioxidants. J. Nutr. 2001, 131, 2248–2251. [Google Scholar] [PubMed]
- Sarkar, A.; Bhaduri, A. black tea is a powerful chemopreventor of reactive oxygen and nitrogen species: Comparison with its individual catechin constituents and green tea. Biochem. Biophys. Res. Commun. 2001, 284, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Dreger, H.; Lorenz, M.; Kehrer, A.; Baumann, G.; Stangl, K.; Stangl, V. Characteristics of catechin- and theaflavin-mediated cardioprotection. Exp. Biol. Med. Maywood NJ 2008, 233, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K.; Chen, P.C.; Ho, C.T.; Lin-Shiau, S.Y. Inhibition of xanthine oxidase and suppression of intracellular reactive oxygen species in HL-60 cells by theaflavin-3,3′-digallate, (−)-epigallocatechin-3-gallate, and propyl gallate. J. Agric. Food Chem. 2000, 48, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Iglesias, A.; Quesada, H.; Díaz, S.; Pajuelo, D.; Bladé, C.; Arola, L.; Josepa Salvadó, M.; Mulero, M. DHA sensitizes FaO cells to tert-BHP-induced oxidative effects. Protective role of EGCG. Food Chem. Toxicol. 2013, 62, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schmitz, H.J.; Baumgart, A.; Guedon, D.; Netsch, M.I.; Kreuter, M.H.; Schmidlin, C.B.; Schrenk, D. Toxicity of green tea extracts and their constituents in rat hepatocytes in primary culture, Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2005, 43, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; di Sotto, A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Lee, M.J.; Diamond, L.; Ju, J.; Hong, J.; Bose, M.; Newmark, H.L.; Yang, C. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos. Biol. Fate Chem. 2006, 34, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- De Sousa, G.; Dou, M.; Barbe, D.; Lacarelle, B.; Placidi, M.; Rahmani, R. Freshly isolated or cryopreserved human hepatocytes in primary culture: Influence of drug metabolism on hepatotoxicity. Toxicol. In Vitro 1991, 5, 483–486. [Google Scholar] [CrossRef]
- Fautrel, A.; Chesne, C.; Guillouzo, A.; de Sousa, G.; Placidi, M.; Rahmani, R.; Braut, F.; Pichon, J.; Hoellinger, H.; Vintézou, P.; et al. A multicentre study of acute in vitro cytotoxicity in rat liver cells. Toxicol. In Vitro 1991, 5, 543–547. [Google Scholar] [CrossRef]
- Peyre, L.; Rouimi, P.; de Sousa, G.; Héliès-Toussaint, C.; Carré, B.; Barcellini, S.; Chagnon, M.C.; Rahmani, R. Comparative study of bisphenol A and its analogue bisphenol S on human hepatic cells: A focus on their potential involvement in nonalcoholic fatty liver disease. Food Chem. Toxicol. 2014, 70, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braud, L.; Peyre, L.; De Sousa, G.; Armand, M.; Rahmani, R.; Maixent, J.-M. Effect of Brewing Duration on the Antioxidant and Hepatoprotective Abilities of Tea Phenolic and Alkaloid Compounds in a t-BHP Oxidative Stress-Induced Rat Hepatocyte Model. Molecules 2015, 20, 14985-15002. https://doi.org/10.3390/molecules200814985
Braud L, Peyre L, De Sousa G, Armand M, Rahmani R, Maixent J-M. Effect of Brewing Duration on the Antioxidant and Hepatoprotective Abilities of Tea Phenolic and Alkaloid Compounds in a t-BHP Oxidative Stress-Induced Rat Hepatocyte Model. Molecules. 2015; 20(8):14985-15002. https://doi.org/10.3390/molecules200814985
Chicago/Turabian StyleBraud, Laura, Ludovic Peyre, Georges De Sousa, Martine Armand, Roger Rahmani, and Jean-Michel Maixent. 2015. "Effect of Brewing Duration on the Antioxidant and Hepatoprotective Abilities of Tea Phenolic and Alkaloid Compounds in a t-BHP Oxidative Stress-Induced Rat Hepatocyte Model" Molecules 20, no. 8: 14985-15002. https://doi.org/10.3390/molecules200814985





