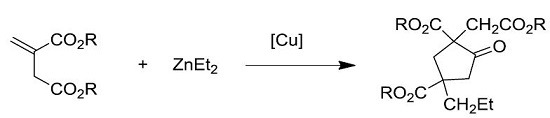
Copper-Catalyzed Dimerization/Cyclization of Itaconates
Abstract
:1. Introduction


2. Results and Discussion
| Entry | Cat. | Ligand | Temp (°C) | Time (h) | Yield b (%) | cis/trans c |
|---|---|---|---|---|---|---|
| 1 | - | - | 25 | 2.5 | 40 | 38:62 |
| 2 | Cu(OAc)2·2H2O | - | 25 | 4.5 | 56 | 52:48 |
| 3 | CuCl | - | 25 | 2.0 | 55 | 46:54 |
| 4 | CuBr | - | 25 | 2.0 | 65 | 55:45 |
| 5 | CuI | - | 25 | 2.0 | 75 | 37:63 |
| 6 | CuI | - | −15 | 2.0 | 61 | 30:70 |
| 7 | CuI | - | −30 | 2.0 | 68 | 27:73 |
| 8 | CuFL | - | 25 | 2.0 | 72 | 61:39 |
| 9 | CuFL | - | 0 | 2.5 | 73 | 57:43 |
| 10 | CuFL | - | −30 | 9.0 | 67 | 62:38 |
| 11 | CuFL | - | −58 | 21.0 | 40 | 56:44 |
| 12 | CuFL | - | −78 | 21.0 | 1 | - |
| 13 | CuFL d | - | 25 | 4.0 | 53 | 55:45 |
| 14 | CuFL e | - | 25 | 2.5 | 74 | 62:38 |
| 15 | CuFL | DPPP f | 25 | 2.0 | 38 | 53:47 |
| 16 | CuFL | DPBen f | 25 | 2.0 | 89 | 62:38 |
| 17 | CuFL | DPEphos f | 25 | 2.0 | 88 | 63:37 |
| 18 | CuFL | Xantphos f | 25 | 2.0 | 95(85 g) | 62:38 |
| 19 | CuFL h | Xantphos | 25 | 2.5 | 98 | 64:36 |
| Entry | 1 (R) | Time (h) | Yield (%) b | cis/trans c |
|---|---|---|---|---|
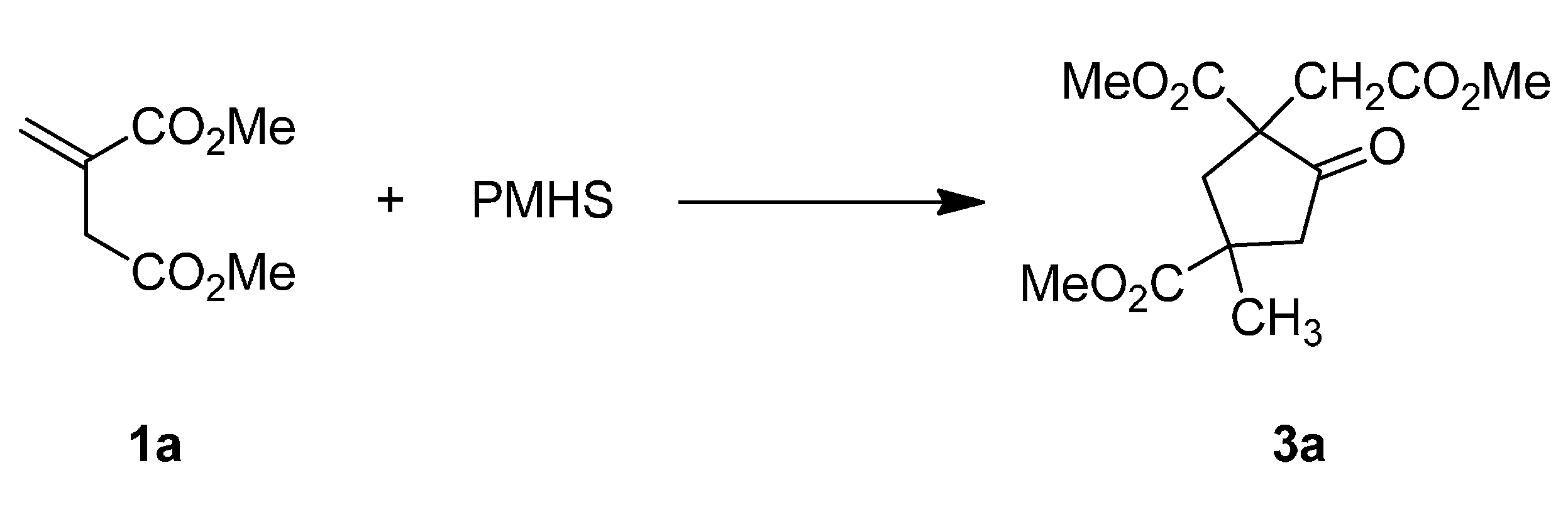
| 1 | 1a (Me) | 2 | 85 | 62:38 |
| 2 | 1b (Et) | 2 | 71 | 73:27 |
| 3 | 1c (n-Bu) | 15 | 79 | 86:14 |
| 4 | 1d (i-Pr) | 18 | 72 | 72:28 d |
3. Experimental Section
3.1. General Information
3.2. General Procedure for the Reactions
Reductive Dimerization of 1a
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Le Notre, J.; van Mele, D.; Frost, C.G. A New Method for Constructing Quaternary Carbon Centres: Tandem Rhodium-Catalysed 1,4-Addition/Intramolecular Cyclisation. Adv. Synth. Catal. 2007, 349, 432–440. [Google Scholar] [CrossRef]
- Kitamura, M.; Miki, T.; Nakano, K.; Noyori, R. 1,4-Addition of Diorganozincs to α,β-Unsaturated Ketones Catalyzed by a Copper(I)-Sulfonamide Combined System. Bull. Chem. Soc. Jpn. 2000, 73, 999–1014. [Google Scholar] [CrossRef]
- Choi, Y.H.; Choi, J.Y.; Yang, H.Y.; Kim, Y.H. Copper-Catalyzed Conjugate Addition on Macrocyclic, Cyclic, and Acyclic Enones with a Chiral Phosphoramidite Ligand Having a C2-Symmetric Amine Moiety. Tetrahedron Asymmetry 2002, 13, 801–804. [Google Scholar] [CrossRef]
- Wilsily, A.; Fillion, E. Asymmetric Synthesis of Carboxylic Acid Derivatives Having an All-Carbon α-Quaternary Center through Cu-Catalyzed 1,4-Addition of Dialkylzinc Reagents to 2-Aryl Acetate Derivatives. Org. Lett. 2008, 10, 2801–2804. [Google Scholar] [CrossRef] [PubMed]
- Ebisu, Y.; Kawamura, K.; Hayashi, M. Enantioselective Copper-Catalyzed 1,4-Addition of Dialkylzincs to Enones Using a Novel N,N,P-Cu(II) Complex. Tetrahedron Asymmetry 2012, 23, 959–964. [Google Scholar] [CrossRef]
- Arink, A.M.; Braam, T.W.; Keeris, R.; Jastrzebski, J.T.B.H.; Benhaim, C.; Rosset, S.; Alexakis, A.; van Koten, G. Copper(I) Thiolate Catalysts in Asymmetric Conjugate Addition Reactions. Org. Lett. 2004, 6, 1959–1962. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.; Jiang, L.; Li, Z.; Zhao, D. Asymmetric Conjugate Addition to Cyclic Enone Catalyzed by Cu-NHC Complexes with C2 Symmetry. Chin. J. Chem. 2011, 29, 973–977. [Google Scholar] [CrossRef]
- Li, J.; You, S.; Cai, M. Synthesis of Tetrasubstituted Olefins by Stereoselective Allylzincation-Negishi Tandem Reaction of Acetylenic Sulfones. J. Chem. Res. 2008, 8, 429–431. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhu, S.Z. Reformatsky-Type Aldol Reactions of 4-Bromo-4,4-Difluoroacetoacetate with Aldehydes and Ketones. Tetrahedron Lett. 2001, 42, 5741–5744. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Kagayama, A.; Igarashi, K.; Shiina, I. Diastereoselective Aldol Reaction of α-Bromo Ketones with Aliphatic Aldehydes by Using Titanium(II) Chloride and Copper. Chem. Lett. 1999, 1157–1158. [Google Scholar] [CrossRef]
- Du, Y.; Xu, L.W.; Shimizu, Y.; Oisaki, K.; Kanai, M.; Shibasaki, M. Asymmetric Reductive Mannich Reaction to Ketimines Catalyzed by a Cu(I) Complex. J. Am. Chem. Soc. 2008, 130, 16146–16147. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Blake, A.J.; Koovits, P.J.; Stepney, G.J. Diastereoselective Reductive Nitro-Mannich Reactions. J. Org. Chem. 2012, 77, 4711–4724. [Google Scholar] [CrossRef] [PubMed]
- Sakanishi, K.; Itoh, S.; Sugiyama, R.; Nishimura, S.; Kakeya, H.; Iwabuchi, Y.; Kanoh, N. Total Synthesis of the Proposed Structure of Heronamide C. Eur. J. Org. Chem. 2014, 2014, 1376–1380. [Google Scholar] [CrossRef]
- Bausch, C.C.; Johnson, J.S. Conjugate Addition/Ireland-Claisen Rearrangements of Allyl Fumarates: Simple Access to Terminally Differentiated Succinates. J. Org. Chem. 2008, 73, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Yuan, L.; Jiang, L.; Li, Z.; Li, Z. Copper Hydride-Catalyzed Reductive Aldol Addition-Lactonization Domino Reactions of α,β-Unsaturated Diesters. Synlett 2014, 25, 724–728. [Google Scholar] [CrossRef]
- Li, Z.; Feng, Y.; Li, Z.; Jiang, L. Copper-Catalyzed Three-Component Tandem Reactions for the Synthesis of β-Carboalkoxy-γ-Lactams. Synlett 2014, 25, 2899–2902. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yokota, K.; Takada, Y. Cyclodimerization of Dimethyl Methylenesuccinate by Alkylaluminum Compounds. Chem. Lett. 1984, 13, 543–546. [Google Scholar] [CrossRef]
- Tolstikov, G.A.; Miftakhov, M.S.; Akbutina, F.A. Key Lactone for 11-Methylprostaglandins. Zh. Org. Khim. 1985, 21, 674–675. [Google Scholar]
- Johnson, F.; Paul, K.G.; Favara, D.; Ciabatti, R.; Guzzi, U. Prostaglandins. 2. Synthesis of Prostaglandin F2.α. In Optically Active Form from Chiral Precursors. J. Am. Chem. Soc. 1982, 104, 2190–2198. [Google Scholar] [CrossRef]
- Magriotis, P.A.; Johnson, F. Steroids. 3. A New Synthetic Approach to Optically Active Steroids. Total Synthesis of (+)-18-Hydroxyestrone. J. Org. Chem. 1984, 49, 1460–1461. [Google Scholar] [CrossRef]
- Node, M.; Nakamura, D.; Nishide, K.; Inoue, T. A Facile Asymmetric Synthesis of Corey Lactone Utilizing C2-Symmetric Dimethyl 3,7-Dihydroxy-cis-bicyclo[3.3.0]octan-2,6-diene-2,6-dicarboxylate. Heterocycles 1997, 46, 535–540. [Google Scholar] [CrossRef]
- Mitani, M.; Pakjamsai, C.; Tsuchida, T.; Kudoh, H. Oligomerization of Acrylic Acid Derivatives by a Reaction with the Alkylcopper(I)-Phosphine Complex. J. Chem. Res. 2000, 4–5. [Google Scholar] [CrossRef]
- Cis- and trans- refer to the relationships of the two carboxylates linked to the cyclopentanone.
- The structure of the less polar product is assigned as trans-2a, in which the protons of CH2CO2CH3 (δ = 3.15, 2.54 ppm) have weaker or no correlations with the CH2CH2CH3 (δ = 1.88, 1.71 ppm) in the NOESY spectrum. For the more polar product, the protons of CH2CO2CH3 (δ = 2.96, 2.91 ppm) have correlations with the signals of CH2CH2CH3 (δ = 1.92, 1.56 ppm). Therefore, There is a cis-realationship between CH2CH2CH3 and CH2CO2CH3, and the structure of the more polar product is deduced as cis-2a.
- Mori, A.; Fujita, A.; Nishihara, Y.; Hiyama, T. Copper(I) Salt Mediated 1,4-Reduction of α,β-Unsaturated Ketones Using Hydrosilanes. Chem. Commun. 1997, 2159–2160. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, L.; Li, Z.; Chen, H. Copper Hydride-Catalyzed Conjugate Reduction-Aldol Addition Domino Reaction of α,β-Unsaturated Carboxylates with Ketones. Chin. J. Chem. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Zheng, A.J.; Shan, F.J.; Li, Z.N.; Li, Z.C.; Jiang, L. Copper Hydride-Catalyzed Reduction of Electron-Deficient Olefins. Chem. Pap. 2013, 67, 1271–1276. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yokota, K. Oligomerization of Allyl Methacrylate in the Presence of Ethylaluminum Compounds and Isolation of Its Unimer and Dimer. Polym. J. 1993, 25, 639–643. [Google Scholar] [CrossRef]
- Brown, M.K.; Degrado, S.J.; Hoveyda, A.H. Highly Enantioselective Cu-Catalyzed Conjugate Additions of Dialkylzinc Reagents to Unsaturated Furanones and Pyranones: Preparation of Air-Stable and Catalytically Active Cu-Peptide Complexes. Angew. Chem. Int. Ed. 2005, 44, 5306–5310. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.A.; Bokovic, Z.V.; Lipshutz, B.H. (BDP)CuH: A “Hot” Stryker’s Reagent for Use in Achiral Conjugate Reductions. Org. Lett. 2008, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ou, J.; Miesch, M.; Chiu, P. Conjugate Reduction and Reductive Aldol Cyclization of α,β-Unsaturated Thioesters Catalyzed by (BDP)CuH. Org. Biomol. Chem. 2011, 9, 6143–6147. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 2a–2d are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, R.; Jiang, L.; Li, Z. Copper-Catalyzed Dimerization/Cyclization of Itaconates. Molecules 2015, 20, 15023-15032. https://doi.org/10.3390/molecules200815023
Li Z, Li R, Jiang L, Li Z. Copper-Catalyzed Dimerization/Cyclization of Itaconates. Molecules. 2015; 20(8):15023-15032. https://doi.org/10.3390/molecules200815023
Chicago/Turabian StyleLi, Zhiqiang, Ruirui Li, Lan Jiang, and Zhengning Li. 2015. "Copper-Catalyzed Dimerization/Cyclization of Itaconates" Molecules 20, no. 8: 15023-15032. https://doi.org/10.3390/molecules200815023