Novel Metal Nanomaterials and Their Catalytic Applications
Abstract
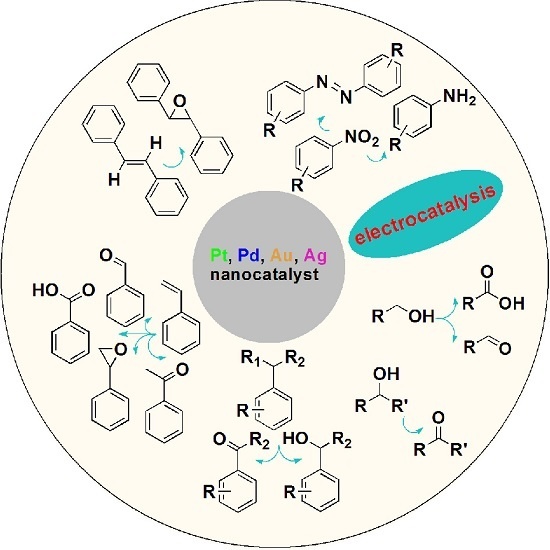
:1. Introduction
2. Pt Nanocomplexes and Catalytic Applications in Organic Reactions
2.1. FePt@Cu NWs and the Catalytic Epoxidation

2.2. Pt@Fe2O3 NWs and the Selective Oxidation


2.3. Bimetallic Pt@Ir Nanocomplexes and the Catalytic Hydrogenation


3. Bimetallic Pt-M (Au and Pd) Nanocomplexes and Their Electro-Catalysis
3.1. Pt-Au Heterostructures and the Oxygen-Reduction Reaction

3.2. Au-Pt Bimetallic Nanocomplexes and the Electro-Catalysis

3.3. Pt/Pd Bimetallic Nanodendrites and the Electro-Catalysis

4. Au, Pd, Ag Nanostructures and Catalytic Applications in Organic Reactions
4.1. Au NWs and the Selective Oxidation

| Entry | Solvent | Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|---|
 |  |  | |||
| 1 | DMF | 23.2 | 27.9 | 63.3 | 8.8 |
| 2 | 1,4-dioxane | 81.7 | 38.5 | 53.9 | 1.6 |
| 3 | m-xylene | 22.7 | 70.5 | 19.2 | 10.3 |
| 4 | p-xylene | 39.5 | 62.5 | 23.8 | 13.7 |
| 5 | chlorobenzene | 3.9 | 69.3 | 30.7 | - |
| 6 | heptane | 28.6 | 50.6 | 5.7 | 3.8 |
| 7 | toluene | 17.0 | 87.2 | 9.9 | 0.9 |
| Entry | R | R1 | R2 | Conversion (%) | Selectivity (%) | |
|---|---|---|---|---|---|---|
 |  | |||||
| 1 | H | H | H | 1.3 | 95.2 | 4.8 |
| 2 | o-CH3 | H | H | 4.3 | 62.1 | 37.9 |
| 3 | m-CH3 | H | H | 1.3 | 100 | - |
| 4 | p-CH3 | H | H | 3.4 | 82.4 | 17.6 |
| 5 | p-Cl | H | H | 1.0 | 100 | - |
| 6 | p-OCH3 | H | H | 0.6 | 100 | - |
| 7 | H | H | CH3 | 21.1 | 100 | - |
| 8 | p-NO2 | H | CH3 | 23.1 | 83.5 | 16.5 |
| 9 | p-OCH3 | H | CH3 | 1.3 | 70.8 | 29.2 |
| 10 | H | CH3 | CH3 | 47.9 | 62.9 | 37.1 |
4.2. CuO@Ag NWs and the Selective Oxidation

4.3. Pd Nanocatalysts and the Reductive Coupling Reactions

5. Perspectives and Challenges
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yadav, G.D.; Nair, J.J. Sulfated zirconia and its modified versions as promising catalysts for industrial processes. Microporous Mesoporous Mater. 1999, 33, 1–48. [Google Scholar] [CrossRef]
- Vermeiren, W.; Gilson, J.-P. Impact of zeolites on the petroleum and petrochemical industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Chen, M.Q.; Wu, L.X.; Wang, W.K.; Fan, M.Y.; Zhang, Y.X.; Tang, H.T. A Catalytic Cracking Method and Device for Double-Riser. Patent CN 104513673, 15 April 2015. [Google Scholar]
- Sharifzadeh, M.; Wang, L.; Shah, N. Decarbonisation of olefin processes using biomass pyrolysis oil. Appl. Energy 2015, 149, 404–414. [Google Scholar] [CrossRef]
- Wolczanski, P.T.; Chirik, P.J. A career in catalysis: John E. bercaw. ACS Catal. 2015, 5, 1747–1757. [Google Scholar] [CrossRef]
- Tanaka, K.-I. Unsolved problems in catalysis. Catal. Today 2010, 154, 105–112. [Google Scholar] [CrossRef]
- Dutta, D.K.; Borah, B.J.; Sarmah, P.P. Recent advances in metal nanoparticles stabilization into nanopores of montmorillonite and their catalytic applications for fine chemicals synthesis. Catal. Rev. 2015, 57, 257–305. [Google Scholar] [CrossRef]
- Yasukawa, T.; Suzuki, A.; Miyamura, H.; Nishino, K.; Kobayashi, S. Chiral metal nanoparticle systems as heterogeneous catalysts beyond homogeneous metal complex catalysts for asymmetric addition of arylboronic acids to α,β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 2015, 137, 6616–6623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shen, X.T.; Zheng, Z.P.; Ma, Y.Y.; Qu, Y.Q. 3D graphene/nylon rope as a skeleton for noble metal nanocatalysts for highly efficient heterogeneous continuous-flow reactions. J. Mater. Chem. A 2015, 3, 10504–10511. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Gao, W.; Qu, Y.Q. Insights into the effects of surface properties of oxides on the catalytic activity of Pd for C-C coupling reactions. Nanoscale 2015, 7, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.J.; Zhou, Z.H.; Song, S.Q.; Li, W.Z.; Sun, G.Q.; Tsiakaras, P.; Xin, Q. Pt based anode catalysts for direct ethanol fuel cells. Appl. Catal. B Environ. 2003, 46, 273–285. [Google Scholar] [CrossRef]
- Tripkovic, A.V.; Popovic, K.D.; Grgur, B.N.; Blizanac, B.; Ross, P.N.; Markovic, N.M. Methanol electrooxidation on supported Pt and PtRu catalysts in acid and alkaline solutions. Electrochim. Acta 2002, 47, 3707–3714. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jeong, K.-J.; Dong, Y.J.; Han, J.H.; Lim, T.-H.; Lee, Jae-S.; Sung, Y.-E. Electro-oxidation of methanol and formic acid on PtRu and PtAu for direct liquid fuel cells. J. Power Sources 2006, 163, 71–75. [Google Scholar] [CrossRef]
- Shen, Y.; Xiao, K.J.; Xi, J.Y.; Qiu, X.P. Comparison study of few-layered graphene supported platinum and platinum alloys for methanol and ethanol electro-oxidation. J. Power Sources 2015, 278, 235–244. [Google Scholar] [CrossRef]
- Xiao, M.L.; Feng, L.G.; Zhu, J.B.; Liu, C.P.; Xing, W. Rapid synthesis of a PtRu nano-sponge with different surface compositions and performance evaluation for methanol electrooxidation. Nanoscale 2015, 7, 9467–9471. [Google Scholar] [CrossRef] [PubMed]
- Varghese, O.K.; Paulose, M.; LaTempa, T.J.; Grimes, C.A. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett. 2009, 9, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Barman, B.K.; Nanda, K.K. Rapid reduction of GO by hydrogen spill-over mechanism by in situ generated nanoparticles at room temperature and their catalytic performance towards 4-nitrophenol reduction and ethanol oxidation. Appl. Catal. A Gen. 2015, 491, 45–51. [Google Scholar] [CrossRef]
- Noh, J.-H.; Meijboom, R. Synthesis and catalytic evaluation of dendrimer-templated andreverse microemulsion Pd and Pt nanoparticles in the reductionof 4-nitrophenol: The effect of size and synthetic methodologies. Appl. Catal. A Gen. 2015, 497, 107–120. [Google Scholar] [CrossRef]
- Song, P.; He, L.-L.; Wang, A.-J.; Mei, L.-P.; Zhong, S.-X.; Chen, J.-R.; Feng, J.-J. Surfactant-free synthesis of reduced graphene oxide supported porous PtAu alloyed nanoflowers with improved catalytic activity. J. Mater. Chem. A 2015, 3, 5321–5327. [Google Scholar] [CrossRef]
- Wang, J.Q.; Geng, H.B.; Li, X.M.; Pan, Y.; Gu, H.W. Novel ultra-thin platinum nanowires and their catalytic applications. Curr. Org. Chem. 2015, 19. [Google Scholar] [CrossRef]
- Jorgenson, K.A. Transition-metal-catalyzed epoxidations. Chem. Rev. 1989, 89, 431–458. [Google Scholar] [CrossRef]
- Hu, L.; Shi, L.Y.; Hong, H.Y.; Li, M.; Bao, Q.Y.; Tang, J.X.; Ge, J.F.; Lu, J.M.; Cao, X.Q.; Gu, H.W. Catalytic epoxidation of stilbene with FePt@Cu nanowires and molecular oxygen. Chem. Commun. 2010, 46, 8591–8593. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.Y.; Hu, L.; Li, M.; Zheng, J.W.; Sun, X.H.; Lu, X.H.; Cao, X.Q.; Lu, J.M.; Gu, H.W. Preparation of Pt@Fe2O3 nanowires and their catalysis of selective oxidation of olefins and alcohols. Chem. Eur. J. 2011, 17, 8726–8730. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hou, Y.L.; Kim, J.M.; Sun, S.H. A general strategy for synthesizing FePt nanowires and nanorods. Angew. Chem. Int. Ed. 2007, 46, 6333–6335. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Mandimutsira, B.S.; Todd, R.; Ramdhanie, B.; Fox, J.P.; Goldberg, D.P. Catalytic sulfoxidation and epoxidation with a Mn(III) triazacorrole: Evidence for a “third oxidant” in high-valent porphyrinoid oxidations. J. Am. Chem. Soc. 2004, 126, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.L.; Wang, Z.; Li, X.Q.; Zhang, C. A simple method for epoxidation of olefins using sodium chlorite as an oxidant without a catalyst. J. Org. Chem. 2005, 70, 9610–9613. [Google Scholar] [CrossRef] [PubMed]
- Adam, W.; Roschmann, K.J.; Saha-Möller, C.R.; Seebach, D. cis-Stilbene and (1α,2β,3α)-(2-ethenyl-3-methoxycyclopropyl)benzene as mechanistic probes in the MnIII(salen)-catalyzed epoxidation: Influence of the oxygen source and the counterion on the diastereoselectivity of the competitive concerted and radical-type oxygen transfer. J. Am. Chem. Soc. 2002, 124, 5068–5073. [Google Scholar] [PubMed]
- Qi, X.H.; Li, M.L.; Kuang, Y.; Wang, C.; Cai, Z.; Zhang, J.; You, S.S.; Yin, M.Z.; Wan, P.B.; Luo, L.; et al. Controllable assembly and separation of colloidal nanoparticles through a one-tube synthesis based on density gradient centrifugation. Chem. Eur. J. 2015, 21, 7211–7216. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Y.; Hu, L.; Wang, J.Q.; Cao, X.Q.; Gu, H.W. Highly efficient synthesis of N-substituted isoindolinones and phthalazinones using Pt nanowires as catalysts. Org. Lett. 2012, 14, 1876–1879. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Wang, J.Q.; Cao, X.Q.; Li, X.M.; Gu, H.W. Selective synthesis of secondary amines from nitriles using Pt nanowires as a catalyst. Chem. Commun. 2014, 50, 3512–3515. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Yu, W.-J.; He, L.; Sun, H.; Cao, Y.; He, H.-Y.; Fan, K.-N. A green and efficient oxidation of alcohols by supported gold catalysts using aqueous H2O2 under organic solvent-free conditions. Green Chem. 2009, 11, 756–759. [Google Scholar]
- Hutchings, G.J. Heterogeneous catalysts—Discovery and design. J. Mater. Chem. 2009, 19, 1222–1235. [Google Scholar]
- Zhou, J.; Hua, Z.; Cui, X.; Ye, Z.; Cui, F.; Shi, J. Hierarchical mesoporous TS-1 zeolite: A highly active and extraordinarily stable catalyst for the selective oxidation of 2,3,6-trimethylphenol. Chem. Commun. 2010, 46, 4994–4996. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.Z.; White, M.A.; Sham, T.K.; Pincock, J.A.; Doucet, R.J.; Rao, K.V.R.; Robertson, K.N.; Cameron, T.S. Zeolite-confined nano-RuO2: A green, selective, and efficient catalyst for aerobic alcohol oxidation. J. Am. Chem. Soc. 2003, 125, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Fan, M.H. Reaction kinetics for the catalytic oxidation of sulfur dioxide with microscale and nanoscale iron oxides. Ind. Eng. Chem. Res. 2007, 46, 80–86. [Google Scholar] [CrossRef]
- Koyuncu, D.D.E.; Yasyerli, S. Selectivity and stability enhancement of iron oxide catalyst by ceria incorporation for selective oxidation of h2s to sulfur. Ind. Eng. Chem. Res. 2009, 48, 5223–5229. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Cademartiri, L.; Ozin, G.A. Ultrathin nanowires—A materials chemistry perspective. Adv. Mater. 2009, 21, 1013–1020. [Google Scholar] [CrossRef]
- Terry, T.J.; Dubois, G.; Murphy, A.; Stack, T.D.P. Site isolation and epoxidation reactivity of a templated ferrous bis(phenanthroline) site in porous silica. Angew. Chem. 2007, 119, 963–965. [Google Scholar] [CrossRef]
- Ge, D.H.; Wang, J.Q.; Geng, H.B.; Lu, S.L.; Wang, D.T.; Li, X.M.; Zhao, X.L.; Cao, X.Q.; Gu, H.W. Facile synthesis of copper-based metal oxide nanoparticles with exceptional catalytic activity for the selective oxidation of styrenes into benzaldehydes. ChemPlusChem 2015, 80, 511–515. [Google Scholar] [CrossRef]
- Ho, K.P.; Wong, W.L.; Lam, K.M.; Lai, C.P.; Chan, T.H.; Wong, K.Y. A Simple and effective catalytic system for epoxidation of aliphatic terminal alkenes with manganese(II) as the catalyst. Chem. Eur. J. 2008, 14, 7988–7996. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Domine, M.E. Gold supported on a mesoporous CeO2 matrix as an efficient catalyst in the selective aerobic oxidation of aldehydes in the liquid phase. Chem. Commun. 2005, 4042–4044. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cao, X.Q.; Ge, D.H.; Hong, H.Y.; Guo, Z.Q.; Chen, L.; Sun, X.H.; Tang, J.X.; Zheng, J.W.; Lu, J.M.; et al. Ultrathin platinum nanowire catalysts for direct C-N coupling of carbonyls with aromatic nitro compounds under 1 bar of hydrogen. Chem. Eur. J. 2011, 17, 14283–14287. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.D.; Xu, J.H.; Wan, J.W.; Xie, B.; Ma, X.B. Facile one-pot fabrication of a silica gel-supported chiral phase-transfer catalyst-N-(2-cyanobenzyl)-O(9)-allyl-cinchonidinium salt. Catal. Sci. Technol. 2015, 5, 2141–2148. [Google Scholar] [CrossRef]
- Ramana, T.; Punniyamurthy, T. Copper-catalyzed domino one-pot synthesis of 2-(arylselanyl)arylcyanamides. Eur. J. Org. Chem. 2011, 25, 4756–4759. [Google Scholar] [CrossRef]
- Rout, L.; Saha, P.; Jammi, S.; Punniyamurthy, T. Efficient copper(I)-catalyzed C-S cross coupling of thiols with aryl halides in water. Eur. J. Org. Chem. 2008, 2008, 640–643. [Google Scholar] [CrossRef]
- Wang, J.Q.; Ge, D.H.; Cao, Q.X.; Tang, M.H.; Pan, Y.; Gu, H.W. A facile synthesis of Pt@Ir zigzag bimetallic nanocomplexes for hydrogenation reactions. Chem. Commun. 2015, 51, 9216–9219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Y.; Ikushima, Y.; Arai, M. Hydrogenation of nitrobenzene with supported platinum catalysts in supercritical carbon dioxide: Effects of pressure, solvent, and metal particle size. J. Catal. 2004, 224, 479–483. [Google Scholar] [CrossRef]
- Lia, C.-H.; Yua, Z.-X.; Yao, K.-F.; Ji, S.-F.; Liang, J. Nitrobenzene hydrogenation with carbon nanotube-supported platinum catalyst under mild conditions. J. Mol. Catal. A Chem. 2005, 226, 101–105. [Google Scholar] [CrossRef]
- Corma, A.; Serna, P. Preparation of substituted anilines from nitro compounds by using supported gold catalysts. Nat. Protoc. 2007, 1, 2590–2595. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hu, L.; Cao, X.Q.; Hong, H.Y.; Lu, J.M.; Gu, H.W. Direct hydrogenation of nitroaromatics and one-pot amidation with carboxylic acids over platinum nanowires. Chem. Eur. J. 2011, 17, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cao, X.Q.; Chen, L.; Zheng, J.W.; Lu, J.M.; Sun, X.H.; Gu, H.W. Highly effcient synthesis of aromatic azos catalyzed by unsupported ultra-thin Pt nanowires. Chem. Commun. 2012, 48, 3445–3447. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Li, C.; Wang, J.Q.; Pan, Y.; Cao, X.Q.; Gu, H.W. Interfacial hydrogenation and deamination of nitriles to selectively synthesize tertiary amines. Chem. Commun. 2014, 50, 11110–11113. [Google Scholar] [CrossRef] [PubMed]
- Highfield, J. Advances and recent trends in heterogeneous photo(electro)-catalysis for solar fuels and chemicals. Molecules 2015, 20, 6739–6793. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.Q.; Zhang, J.C.; Zhang, R.C.; Shi, H.Z.; Guo, X.L.; Guo, Y.Y.; Guo, X.Y.; Cai, S.S.; Zhang, D.J. Electrochemical and electrocatalytic properties of a stable Cu-based metal-organic framework. Int. J. Electrochem. Sci. 2015, 10, 4899–4910. [Google Scholar]
- Cai, G.-X.; Guo, J.-W.; Wang, J.; Li, S. Negative resistance for methanol electro-oxidation on platinum/carbon (Pt/C) catalyst investigated by an electrochemical impedance spectroscopy. J. Power Sources 2015, 276, 279–290. [Google Scholar] [CrossRef]
- Mallick, R.K.; Thombre, S.B.; Shrivastava, N.K. A critical review of the current collector for passive direct methanol fuel cells. J. Power Sources 2015, 285, 510–529. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, Y.; Chen, H.L.; Wang, Z.G.; Zeng, Z.Y.; Cai, M.Y.; Zhang, Y.F.; Liu, X.W. A water management system for metal-based micro passive direct methanol fuel cells. J. Power Sources 2015, 273, 375–379. [Google Scholar] [CrossRef]
- Yang, Z.H.; Hafez, I.H.; Berber, M.R.; Nakashima, N. An enhanced anode based on polymer-coated carbon black for use as a direct methanol fuel cell electrocatalyst. ChemCatChem 2015, 7, 808–813. [Google Scholar] [CrossRef]
- Wang, C.; Daimon, H.; Sun, S.H. Dumbbell-like Pt-Fe3O4 nanoparticles and their enhanced catalysis for oxygen reduction reaction. Nano Lett. 2009, 9, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Li, Y.W.; Yue, K.; Wang, Z.; Lu, P.T.; Feng, X.Y.; Dong, X.-H.; Zhang, S.; Cheng, S.Z.D.; Zhang, W.-B. Macromolecular structure evolution toward giant molecules of complex structure: Tandem synthesis of asymmetric giant gemini surfactants. Polym. Chem. 2014, 5, 3697–3706. [Google Scholar] [CrossRef]
- Hun, X.; Xie, G.L.; Luo, X.L. Scaling up an electrochemical signal with a catalytic hairpin assembly coupling nanocatalyst label for DNA detection. Chem. Commun. 2015, 51, 7100–7103. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.K.; Selvan, S.T.; Lee, S.S.; Papaefthymiou, G.C.; Kundaliya, D.; Ying, J.Y. Silica-coated nanocomposites of magnetic nanoparticles and quantum dots. J. Am. Chem. Soc. 2005, 127, 4990–4991. [Google Scholar] [CrossRef] [PubMed]
- Costi, R.; Saunders, A.E.; Elmalem, E.; Salant, A.; Banin, U. Visible light-induced charge retention and photocatalysis with hybrid CdSe-Au nanodumbbells. Nano Lett. 2008, 8, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Mokari, T.; Sztrum, C.G.; Salant, A.; Rabani, E.; Banin, U. Formation of asymmetric one-sided metal-tipped semiconductor nanocrystal dots and rods. Nat. Mater. 2005, 4, 855–863. [Google Scholar] [CrossRef]
- Gu, H.W.; Yang, Z.M.; Gao, J.H.; Chang, C.K.; Xu, B. Heterodimers of nanoparticles: Formation at a liquid-liquid interface and particle-specific surface modification by functional molecules. J. Am. Chem. Soc. 2005, 127, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.Y.; Cao, X.Q.; Zhen, J.W.; Shao, H.L.; Gu, H.W.; Lu, J.M.; Ying, J.Y. Facile synthesis of hybrid nanostructures from nanoparticles, nanorods and nanowires. J. Mater. Chem. 2011, 21, 11478–11481. [Google Scholar] [CrossRef]
- Xie, R.G.; Chen, M.Z.; Wang, J.Q.; Mei, S.J.; Pan, Y.; Gu, H.W. Facile synthesis of Au-Pt bimetallic nanocomplexes for direct oxidation of methanol and formic acid. RSC Adv. 2015, 5, 650–653. [Google Scholar] [CrossRef]
- Wu, H.; Mei, S.J.; Cao, X.Q.; Zheng, J.W.; Lin, M.; Tang, J.X.; Ren, F.F.; Du, Y.K.; Pan, Y.; Gu, H.W. Facile synthesis of Pt/Pd nanodendrites for the direct oxidation of methanol. Nanotechnology 2014, 25, 195702. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Daimon, H.; Onodera, T.; Koda, T.; Sun, S.H. A general approach to the size- and shape-controlled synthesis of platinum nanoparticles and their catalytic reduction of oxygen. Angew. Chem. 2008, 120, 3644–3647. [Google Scholar] [CrossRef]
- Nkosi, B.; Adams, M.D.; Coville, N.J.; Hutchings, G.J. Hydrochlorination of acetylene using carbon-supported gold catalysts: A study of catalyst reactivation. J. Catal. 1991, 128, 378–386. [Google Scholar] [CrossRef]
- Lin, M. A dopamine electrochemical sensor based on gold nanoparticles/over-oxidized polypyrrole nanotube composite arrays. RSC Adv. 2015, 5, 9848–9851. [Google Scholar] [CrossRef]
- Sunil Sekhar, A.C.; Ziyad, K.; Soni, Y.; Vinod, C.P. Activity enhancement upon the incorporation of titanium: Au@Ti-SiO2 core-shell nanocatalysts for the CO oxidation reaction. ChemCatChem 2015, 7, 1222–1230. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Supported gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef] [PubMed]
- Lignier, P.; Comotti, M.; Schüth, F.; Rousset, J.L.; Caps, V. Effect of the titania morphology on the Au/TiO2-catalyzed aerobic epoxidation of stilbene. Catal. Today 2009, 141, 355–360. [Google Scholar] [CrossRef]
- Lignier, P.; Mangematin, S.; Morfin, F.; Rousset, J.L.; Caps, V. Solvent and oxidant effects on the Au/TiO2-catalyzed aerobic epoxidation of stilbene. Catal. Today 2008, 138, 50–54. [Google Scholar] [CrossRef]
- Lignier, P.; Morfin, F.; Mangematin, S.; Massin, L.; Rousset, J.L.; Caps, V. Stereoselective stilbene epoxidation over supported gold-based catalysts. Chem. Commun. 2007, 2, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Gajan, D.; Guillois, K.; Delichère, P.; Basset, J.M.; Candy, J.P.; Caps, V.; Copéret, C.; Lesage, A.; Emsley, L. Gold nanoparticles supported on passivated silica: Access to an efficient aerobic epoxidation catalyst and the intrinsic oxidation activity of gold. J. Am. Chem. Soc. 2009, 131, 14667–14669. [Google Scholar] [CrossRef] [PubMed]
- Boualleg, M.; Guillois, K.; Istria, B.; Burel, L.; Veyre, L.; Basset, J.M.; Thieuleux, C.; Caps, V. Highly efficient aerobic oxidation of alkenes over unsupported nanogold. Chem. Commun. 2010, 46, 5361–5363. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cao, X.Q.; Yang, J.H.; Li, M.; Hong, H.Y.; Xu, Q.F.; Ge, J.F.; Wang, L.H.; Lu, J.M.; Chen, L.; et al. Oxidation of benzylic compounds by gold nanowires at 1 atm O2. Chem. Commun. 2011, 47, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.Y.; Tsung, C.K.; Huang, W.Y.; Zhang, X.F.; Yang, P.D. Sub-two nanometer single crystal Au nanowires. Nano Lett. 2008, 8, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Gates, B.; Mayers, B.; Xia, Y.N. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Sun, Y.G.; Yin, Y.D.; Mayers, B.T.; Herricks, T.; Xia, Y.N. Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly(vinyl pyrrolidone). Chem. Mater. 2002, 14, 4736–4745. [Google Scholar] [CrossRef]
- Sun, Y.G.; Tao, Z.L.; Chen, J.; Herricks, T.; Xia, Y.N. Ag nanowires coated with Ag/Pd alloy sheaths and their use as substrates for reversible absorption and desorption of hydrogen. J. Am. Chem. Soc. 2004, 126, 5940–5941. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Wiley, B.J.; Xia, Y.N. One-dimensional nanostructures of metals: Large-scale synthesis and some potential applications. Langmuir 2007, 23, 4120–4129. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.M.; Hu, L.; Jiang, J.; Tang, J.X.; Cao, X.Q.; Gu, H.W. CuO@Ag as a highly active catalyst for the selective oxidation of trans-stilbene and alcohols. Catal. Sci. Technol. 2012, 2, 1146–1149. [Google Scholar] [CrossRef]
- Basu, M.; Sinha, A.K.; Pradhan, M.; Sarkar, S.; Pal, A.; Pal, T. Monoclinic CuO nanoflowers on resin support: Recyclable catalyst to obtain perylene compound. Chem. Commun. 2010, 46, 8785–8787. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.I.; Tsung, C.K.; Huang, W.; Yang, P. Room-temperature formation of hollow Cu2O nanoparticles. Adv. Mater. 2012, 22, 1910–1914. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.P.; Sithambaram, S.; Zhang, Y.S.; Chen, C.H.; Jin, L.; Joesten, R.; Suib, S.L. Novel urchin-like CuO synthesized by a facile reflux method with efficient olefin epoxidation catalytic performance. Chem. Mater. 2009, 21, 1253–1259. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Zheng, X.L. Plasma-enhanced catalytic CuO nanowires for CO oxidation. Nano Lett. 2010, 10, 4762–4766. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Kim, J.; Kwon, H.; Song, H. Gram-scale synthesis of Cu2O nanocubes and subsequent oxidation to CuO hollow nanostructures for lithium-ion battery anode materials. Adv. Mater. 2009, 21, 803–807. [Google Scholar] [CrossRef]
- Chen, C.Q.; Qu, J.; Cao, C.Y.; Niu, F.; Song, W.G. CuO nanoclusters coated with mesoporous SiO2 as highly active and stable catalysts for olefin epoxidation. J. Mater. Chem. 2011, 21, 5774–5779. [Google Scholar] [CrossRef]
- Hu, L.; Cao, X.Q.; Shi, L.Y.; Qi, F.Q.; Guo, Z.Q.; Lu, J.M.; Gu, H.W. A Highly active nano-palladium catalyst for the preparation of aromatic azos under mild conditions. Org. Lett. 2011, 13, 5640–5643. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Hu, L.; Cao, X.Q.; Lu, J.M.; Li, X.M.; Gu, H.W. Catalysis by Pd nanoclusters generated in situ of high-efficiency synthesis of aromatic azo compounds from nitroaromatics under H2 atmosphere. RSC Adv. 2013, 3, 4899–4902. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.Q.; Qi, F.Q.; Hu, L.; Li, X.M.; Cao, X.Q.; Gu, H.W. Synthesis of in-situ surfactant-free Pd nanoparticle catalysts for the synthesis of aromatic azo compounds and for unsaturated bond hydrogenation by hydrogen transfer. Chin. J. Catal. 2013, 34, 2084–2088. [Google Scholar] [CrossRef]
- Ashutosh; Pandey, N.D.; Mehrotra, J.K. Azo dyes as metallochromic indicators. Colourage 1979, 26, 25. [Google Scholar]
- Denizli, A.; Piskin, E. Dye-ligand affinity systems. J. Biochem. Biophys. Methods 2001, 49, 391–416. [Google Scholar] [CrossRef]
- Hoult, J.R.S. Pharmacological and biochemical actions of sulphasalazine. Drugs 1986, 32, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Hanauer, S.B. Systematic review: The pharmacokinetic profiles of oral mesalazine formulations and mesalazine pro-drugs used in the management of ulcerative colitis. Aliment. Pharmacol. Ther. 2003, 17, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Noor, S.; Sharif, Q.M.; Najeebullah, M. Different techniques recently used for the treatment of textile dyeing effluents: A review. J. Chem. Soc. Pak. 2010, 32, 115–124. [Google Scholar]
- Osman, M.Y.; Sharaf, I.A.; Osman, H.M.Y.; El-Khouly, Z.A.; Ahmed, E.I. Synthetic organic food colouring agents and their degraded products: Effects on human and rat cholinesterases. Br. J. Biomed. Sci. 2004, 61, 128–132. [Google Scholar] [PubMed]
- Shimada, C.; Kano, K.; Sasaki, Y.F.; Sato, I.; Tsudua, S. Differential colon DNA damage induced by azo food additives between rats and mice. J. Toxicol. Sci. 2010, 35, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.J.; Ding, K.L.; Wu, T.B.; Zhou, X.S.; Fan, H.L.; Jiang, T.; Wang, Q.; Han, B.X. Shape controlled synthesis of palladium nanocrystals by combination of oleylamine and alkylammonium alkylcarbamate and their catalytic activity. Chem. Commun. 2010, 46, 8552–8554. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.W.; Pei, W.; Ma, X.; Xu, X.; Ren, Y.; Sun, W.; Zuo, L. Enhanced catalytic activity of Pt nanomaterials: From monodisperse nanoparticles to self-organized nanoparticle-linked nanowires. J. Phys. Chem. C 2010, 114, 6909–6913. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, W.-H.; Han, K.W.; Choi, Y.K.; Park, J. Dynamic kinetic resolution of primary amines with a recyclable Pd nanocatalyst for racemization. Org. Lett. 2007, 9, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.P.; Hu, T.J.; Chen, X.R.; Xu, K.L.; Zhang, J.L.; Huang, J. Pd nanoparticles on a porous ionic copolymer: A highly active and recyclable catalyst for Suzuki-Miyaura reaction under air in water. Chem. Commun. 2011, 47, 3592–3594. [Google Scholar] [CrossRef] [PubMed]
- Han, R.R.; Nan, C.S.; Yang, L.; Fan, G.L.; Li, F. Direct synthesis of hybrid layered double hydroxide-carbon composites supported Pd nanocatalysts efficient in selective hydrogenation of citral. RSC Adv. 2015, 5, 33199–33207. [Google Scholar] [CrossRef]
- Liu, B.; Ren, Y.S.; Zhang, Z.H. Aerobic oxidation of 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid in water under mild conditions. Green Chem. 2015, 17, 1610–1617. [Google Scholar] [CrossRef]
- Peng, S.-Y.; Xu, Z.-N.; Chen, Q.-S.; Wang, Z.-Q.; Lv, D.-M.; Sun, J.; Chen, Y.M.; Guo, G.-C. Enhanced stability of Pd/ZnO catalyst for CO oxidative coupling to dimethyl oxalate: Effect of Mg2+ doping. ACS Catal. 2015, 5, 4410–4417. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Gu, H. Novel Metal Nanomaterials and Their Catalytic Applications. Molecules 2015, 20, 17070-17092. https://doi.org/10.3390/molecules200917070
Wang J, Gu H. Novel Metal Nanomaterials and Their Catalytic Applications. Molecules. 2015; 20(9):17070-17092. https://doi.org/10.3390/molecules200917070
Chicago/Turabian StyleWang, Jiaqing, and Hongwei Gu. 2015. "Novel Metal Nanomaterials and Their Catalytic Applications" Molecules 20, no. 9: 17070-17092. https://doi.org/10.3390/molecules200917070
APA StyleWang, J., & Gu, H. (2015). Novel Metal Nanomaterials and Their Catalytic Applications. Molecules, 20(9), 17070-17092. https://doi.org/10.3390/molecules200917070