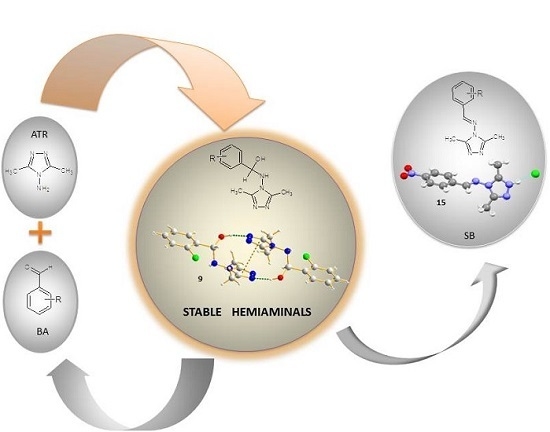
A Study on the Condensation Reaction of 4-Amino-3,5-dimethyl-1,2,4-triazole with Benzaldehydes: Structure and Spectroscopic Properties of Some New Stable Hemiaminals
Abstract
:1. Introduction
2. Results and Discussion

2.1. X-ray Diffraction

| R | Bond Lengths (Å) | Torsion Angle (°) | Dihedral Angle (°) | |||||
|---|---|---|---|---|---|---|---|---|
| C1-C14 | C14-N4 | N4-N3 | C14-O14 | C1t-C3t | N1-N2 | C1-C14-N4-N3 | Phenyl-Triazole | |
| 2-NO2 (2) | 1.529(2) | 1.514(3) | 1.425(2) | 1.363(3) | 1.435(2) | 1.360(3) | 177.1(1) | 19.99(1) |
| 2,4-(NO2)2 (5) | 1.526(3) | 1.461(2) | 1.406(2) | 1.397(3) | 1.481(3) | 1.396(4) | −176.3(2) | 21.27(1) |
| 2-Cl (9) | 1.513(4) | 1.467(4) | 1.410(4) | 1.425(4) | 1.484(4) | 1.403(3) | 179.0(2) | 9.9(2) |
| 4-CHO (10) | 1.510(2) | 1.482(2) | 1.409(2) | 1.401(2) | 1.478(2) | 1.399(2) | 174.3(1) | 28.8(2) |
| 4-NO2 (15) | 1.465(2) | 1.265(2) | 1.418(2) | - | 1.468(3) | 1.367(2) | 179.1(1) | 70.48(2) |

| R | O14–H∙∙∙N2 | Cg1-Cg2 a | |||
|---|---|---|---|---|---|
| D–H | H∙∙∙A | D∙∙∙A | <D–H∙∙∙A | ||
| 2-NO2 (2) | 0.90(2) | 1.87(2) | 2.750(2) | 169(3) | 3.488(1) |
| 2,4-(NO2)2 (5) | 0.85(4) | 2.00(4) | 2.811(3) | 161(3) | 3.371(1) |
| 2-Cl (9) | 0.86(4) | 1.96(4) | 2.771(4) | 156(4) | 3.501(2) |
| 4-CHO (10) | 0.94(1) | 1.77(3) | 2.760(1) | 175(2) | 3.272(2) |
2.2. Spectral Studies
| R1 | Vibration Frequencies (cm−1) | 1H-NMR δ (ppm), J (Hz) | 13C-NMR (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| νOH a | νOH.N a | νNH b | δ(NH) | δ(OH) | δ(CH) | JCH-NH | JCH-OH | δ(C*) | |
| 2-NO2 C6H4 (2) | 3308 | 3114 | 3090 | 7.15 | 6.91 | 5.96 | 8.12 | 5.33 | 79.2 |
| 3-NO2 C6H4 (3) | 3312 | 3115 | 3090 | 7.19 | 6.79 | 5.62 | 7.17 | 5.78 | 83.2 |
| 4-NO2 C6H4 (4) | 3304 | 3079 | 3100 | 7.17 | 6.76 | 5.58 | 7.18 | 5.67 | 83.4 |
| 2,4-(NO2)2 C6H3 (5) | 3305 | 3106 | 3090 | 7.33 | 7.26 | 6.00 | 8.35 | 5.01 | 79.1 |
| 3-NO2,4-Cl C6H3 (6) | 3261 | 3105 | 3080 | 7.20 | 6.86 | 5.57 | 7.44 | 5.72 | 82.7 |
| 3-NO2,4-CH3 C6H3 (7) | 3309 | 3105 | 3070 | 7.12 | 6.70 | 5.54 | 6.87 | 5.69 | 82.6 |
| 2-Cl, 5-NO2 C6H3 (8) | 3309 | 3070 | 3081 | 7.26 | 7.02 | 5.77 | 6.99 | 5.48 | 80.0 |
| 2-Cl C6H4 (9) | 3265 | 3124 | 3081 | 7.05 | 6.60 | 5.76 | 6.29 | 5.15 | 80.8 |
| 4-CHO C6H4 (10) | 3291 | 3077 | 3080 | 7.12 | 6.64 | 5.55 | 6.87 | 5.53 | 83.9 |
| 4-CN C6H4 (11) | 3280 | 3082 | 3084 | 7.13 | 6.70 | 5.53 | 7.06 | 5.72 | 83.6 |
| 4-CF3 C6H4 (12) | 3284 | 3126 | 3082 | 7.11 | 6.66 | 5.55 | 6.87 | 5.53 | 83.7 |
| 3-C5H4N (13) | 3202 | 3125 | 3078 | 7.13 | 6.25 | 5.47 | 6.87 | 5.91 | 82.2 |
| 4-C5H4N (14) | 3191 | 3122 | 3107 | 7.15 | 6.70 | 5.47 | 7.44 | 5.72 | 83.2 |
2.3. Hemiaminal Stability in Solution



2.4. Hemiaminal Formation—Effect of Substituents.

2.5. Hemiaminal Formation—Solvent Effect
2.6. Hemiaminal Formation—Benzaldehyde Concentration and Temperature Effect
| Solvent | HA a | SB a | K b |
|---|---|---|---|
| n-Hexane | 70 | 4 | 17.5 |
| Cyclohexane | 47 | 11 | 4.3 |
| CHCl3 | 47 | 14 | 3.4 |
| Toluene | 33 | 8 | 4.1 |
| CH2Cl2 | 28 | 16 | 1.8 |
| CH3CN | 27 | 4 | 6.8 |
| DMSO | 48 | 12 | 4.0 |
| Pyridine | 35 | 13 | 2.7 |
| Triethylamine | 25 | 19 | 1.3 |
| THF | 30 | 26 | 1.2 |
| 1,4-Dioxane | 6 | 44 | 0.1 |
| 2-Propanol | 38 | 12 | 3.2 |
| H2O | 33 | 8 | 4.1 |
| H2O/CH3CN (V:V) | |||
| 1.5:0.5 | 41 | 10 | 4.1 |
| 1.0:1.0 | 42 | 7 | 6.0 |
| 0.5:1.5 | 41 | 6 | 6.8 |
| 0.4:1.6 | 44 | 6 | 7.3 |
| 0.3:1.7 | 51 | 5 | 10.2 |
| 0.2:1.8 | 51 | 6 | 8.5 |
| 0.1:1.9 | 54 | 7 | 7.7 |

| Temperature °C | 40 | 50 | 60 | 70 | 80 |
|---|---|---|---|---|---|
| K = IHA/ISB a | 8.3 | 6.8 | 5.3 | 3.9 | 2.4 |
2.7. Hemiaminal-Aldehyde Interchange Reaction


3. Experimental Section
3.1. Materials and Physical Measurements
3.2. X-ray Crystallography
3.3. Synthesis of Hemiaminals 2–14
3.4. Synthesis of Imines 15–16
3.5. Reaction of MeATR (1) with 2-Pyridinecarboxaldehyde
3.6. Reaction Survey
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martin, A.; Martin, R. A revive on the antimicrobial activity of 1,2,4-triazolederivatives. Int. J. Life Sci. Pharm. Res. 2014, 3, 321–329. [Google Scholar]
- Singh, G.; Sharma, P.; Dadhwal, S.; Garg, P.; Sharma, S.; Mahajan, N.; Rawal, S. Triazoles-impinging the bioactivities. Int. J. Curr. Res. 2011, 3, 105–118. [Google Scholar]
- Singhal, N.; Sharma, P.K.; Dudhe, R.; Kumar, N. Recent advancement of triazole derivatives and their biological significance. J. Chem. Pharm. Res. 2011, 3, 126–133. [Google Scholar]
- Kumar, P.S.; Mishra, D.; Ghosh, G.; Panda, B.B.; Panda, C.S. Synthesis, characterization and antimicrobial evaluation of some triazole derivative containing thiophen ring. Int. J. ChemTech Res. 2010, 2, 1959–1965. [Google Scholar]
- Paudyal, R.; Jamaluddin, A.; Warren, J.P.; Doyle, S.M.; Robert, S.; Warriner, S.L.; Baker, A. Trafficking modulator TENin1 inhibits endocytosis, causes endomembrane protein accumulation at the pre-vacuolar compartment and impairs gravitropic response in Arabidopsis thaliana. Biochem. J. 2014, 460, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lu, L.; Zhu, M.; Wang, Q.; Li, Y.; Xing, S.; Fu, X.; Gao, Z.; Dong, Y. Mononuclear copper(II) complexes with 3,5-substituted-4-salicylidene-amino-3,5-dimethyl-1,2,4-triazole: Synthesis, structure and potent inhibition of protein tyrosine phosphatases. Dalton Trans. 2011, 40, 6532–6540. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhu, M.; Lu, L.; Feng, S.; Yan, J. Trinuclear-based coordination compounds of Mn(II) and Co(II) with 4-amino-3,5-dimethyl-1,2,4-triazole and azide and thiocyanate anions: Synthesis, structure and magnetic properties. Inorg. Chim. Acta 2011, 370, 102–107. [Google Scholar] [CrossRef]
- Su, C.Y.; Tong, Y.Z.; Yuan, F.C.; Wang, Q.L.; Ma, Y.; Yang, G.M.; Liao, D.Z. Crystal structure, spectroscopy and magnetism of trinuclear and one-dimensional copper(II) complexes. Inorg. Chim. Acta 2014, 423, 545–549. [Google Scholar] [CrossRef]
- Wu, L.C.; Weng, T.C.; Hsu, I.J.; Liu, Y.H.; Lee, G.H.; Lee, J.F.; Wang, Y. Chemical bond characterization of a mixed-valence tri-cobalt complex, Co3(μ-admtrz)4(μ-OH)2(CN)6∙2H2O. Inorg. Chem. 2013, 52, 11023–11033. [Google Scholar] [CrossRef] [PubMed]
- Nockemann, P.; Meyer, G. 3,5-Dimethyl-4-amino-1,2,4-triazole (Dat) as a tridentate ligand in the polymeric cations of [Ag3(Dat)2](NO3)3. Z. Anorg. Allg. Chem. 2007, 633, 2238–2241. [Google Scholar] [CrossRef]
- Wu, B.D.; Bi, Y.G.; Zhou, M.R.; Zhang, T.L.; Yang, L.; Zhou, Z.N.; Zhang, J.G. Stable high-nitrogen energetic trinuclear compounds based on 4-amino-3,5-dimethyl-1,2,4-triazole: Synthesis, structures, thermal and explosive properties. Z. Anorg. Allg. Chem. 2014, 640, 1467–1473. [Google Scholar] [CrossRef]
- Chieb, T.; Belmokre, K.; Benmessaound, M.; Drissi, S.E.H.; Hajjaji, N.; Srhiri, A. The inhibitive effect of 3-methyl-4-amino-1,2,4-triazole on the corrosion of copper-nickel 70–30 in NaCl 3% solution. Mater. Sci. Appl. 2011, 2, 1260–1267. [Google Scholar] [CrossRef]
- Schwärzler, A.; Laus, G.; Kahlenberg, V.; Wurst, K.; Gelbrich, T.; Kreutz, C.; Kopacka, H.; Bonn, G.; Schottenberer, H. Quaternary 4-amino-1,2,4-triazolium salts: Crystal structures of ionic liquids and N-heterocyclic carbene (NHC) complexes. Z. Naturforschung 2009, 64, 603–616. [Google Scholar] [CrossRef]
- Smith, J.M.B.; March, J. March’s Advanced Organic Chemistry, 6th ed.; Wiley Interscience: Hoboken, NJ, USA, 2007; pp. 1281–1284. [Google Scholar]
- Sayer, J.M.; Pinsky, B.; Schonbrunn, A.; Washtien, W. Mechanism of carbinolamine formation. J. Am. Chem. Soc. 1974, 96, 7998–8008. [Google Scholar] [CrossRef]
- Carey, F.A.; Giuliano, R.M. Organic Chemistry, 8th ed.; Mc Graw-Hill: New York, NY, USA, 2011; pp. 746–775. [Google Scholar]
- Namli, H.; Turhan, O. Simultaneous observation of reagent consumption andproduct formation with the kinetics of benzaldehyde and aniline reaction in FTIR liquid cell. Vib. Spectrosc. 2007, 43, 274–283. [Google Scholar] [CrossRef]
- Hooley, R.J.; Iwasawa, T.; Rebek, J., Jr. Detection of reactive tetrahedral intermediates in a deep cavitand with an introverted functionality. J. Am. Chem. Soc. 2007, 129, 15330–15339. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, T.; Hooley, R.J.; Rebek, J., Jr. Stabilization of labile carbonyl addition intermediates by a synthetic receptors. Science 2007, 317, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.; Doonan, C.J.; Yagi, O.M. Postsyntetic modification of a metal-organic framework for stabilization of a hemiaminal and ammonia uptake. Inorg. Chem. 2011, 50, 6853–6855. [Google Scholar] [CrossRef]
- Kawamichi, T.; Haneda, T.; Kawano, M.; Fujita, M. X-ray observation of a transient hemiaminal trapped in a porous network. Nature 2009, 461, 633–635. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Long, S.R.; Lynch, V.M.; Anslyn, E.V. Dynamic multi-component hemiaminal assembly. Chem. Eur. J. 2011, 17, 11017–11023. [Google Scholar] [CrossRef] [PubMed]
- El Mail, I.; Garralda, M.A.; Hernandez, R.; Ibarlucea, L. Reaction of [RhCl(CO)2]2 or [RhCl(COD)]2 with o-(diphenylphosphino)benzaldehyde. Formation of hemiaminals in the subsequent reaction with dihydrazones. J. Organomet. Chem. 2002, 648, 149–154. [Google Scholar] [CrossRef]
- Billard, T.; Langlois, B.R.; Blond, G. Trifluoromethylation of nonenolizable carbonyl compounds with a stable piperazino hemiaminal of trifluoroacetaldehyde. Eur. J. Org. Chem. 2001, 2001, 1467–1471. [Google Scholar] [CrossRef]
- Dolenský, B.; Kvíčala, J.; Paleta, O. Methyl 3,3,3-trifluoropyruvate hemiaminals: Stability and transamination. J. Fluor. Chem. 2005, 126, 745–751. [Google Scholar] [CrossRef]
- Paleta, O.; Dolenský, B.; Paleček, J.; Kvíčala, J. New nucleophilic rearrangement in the mechanism of the three-component domino cyclisation affording fluoroalkylated (pyrrolo)quinazolines. J. Fluor. Chem. 2014, 157, 1–11. [Google Scholar] [CrossRef]
- Suni, V.; Kurup, M.R.P.; Nethaji, M. Unusual isolation of a hemiaminal product from 4-cyclohexyl-3-thiosemicarbazide and di-2-pyridyl ketone: Structural and spectral investigations. J. Mol. Struct. 2005, 749, 177–182. [Google Scholar] [CrossRef]
- Evans, D.A.; Borg, G.; Scheidt, K.A. Remarkably stable tetrahedral intermediates: Carbinols from nucleophilic addition to N-acylpyrroles. Angew. Chem. Int. Ed. 2002, 41, 3188–3191. [Google Scholar] [CrossRef]
- Szostak, M.; Yao, L.; Aube, J. Proximity effect in nucleophilic addition reactions to medium-bridged twisted lactams: Remarkably stable tetrahedral intermediates. J. Am. Chem. Soc. 2010, 132, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- Barys, M.; Ciunik, Z.; Drabent, K.; Kwiecień, A. Stable hemiaminals containing a triazole ring. New J. Chem. 2010, 34, 2605–2611. [Google Scholar] [CrossRef]
- Kwiecień, A.; Barys, M.; Ciunik, Z. Stable hemiaminals with a cyano group and triazole ring. Molecules 2014, 19, 11160–11177. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, W.X.; Ye, B.H.; Lin, J.B.; Chen, X.M. In situ solvothermal generation of 1,2,4-triazolates and related compounds from organonitrile and hydrazine hydrate: A mechanism study. Inorg. Chem. 2007, 46, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Daszkiewicz, M.; Marchewka, M.K. Crystal structure, vibrational and theoretical studies of bis(4-amino-1,2,4-triazolium) hexachloridostannate(IV). J. Mol. Struct. 2004, 705, 177–187. [Google Scholar] [CrossRef]
- Larkin, P.J. Infrared and Raman Spectroscopy; Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011; pp. 124–125. [Google Scholar]
- Berski, S.; Ciunik, L. The mechanism of the formation of the hemiaminal and Schiff base from the benzaldehyde and triazole studied by means of the topological analysis of electron localisation function and catastrophe theory. Mol. Phys. 2015, 113, 765–781. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spectroscopic Methods in Organic Chemistry, 2nd ed.; Thieme Verlag: Stuttgart, Germany, 2007; pp. 112–114. [Google Scholar]
- Feldmann, M.T.; Widicus, S.L.; Blake, G.A.; Kent, D.R., IV; Goddard, W.A., III. Aminomethanol water elimination: Theoretical examination. J. Chem. Phys. 2005, 123, 034304. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Unger, S.H.; Kim, K.H.; Nikaitani, D.; Lien, E.J. Aromatic substituent constants for structure-activity correlations. J. Med. Chem. 1973, 16, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley-VCH Verlag GmbH & Co, KGaA: Weinheim, Germany, 2003; pp. 93–145. [Google Scholar]
- Hall, N.E.; Smith, B.J. Solvation effects on zwitterion formation. J. Phys. Chem. A 1998, 102, 3985–3990. [Google Scholar] [CrossRef]
- Hall, N.E.; Smith, B.J. High-level ab initio molecular orbital calculation of imine formation. J. Phys. Chem. A 1998, 102, 4930–4938. [Google Scholar] [CrossRef]
- Sunoj, R.B.; Anand, M. Mocrosolvated transition state models for improved insight into chemical properties and reaction mechanism. Phys. Chem. Chem. Phys. 2012, 14, 12715–12736. [Google Scholar] [CrossRef] [PubMed]
- Helfferich, F.G. Kinetics of Multistep Reactions, Comprehensive Chemical Kinetics, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2004; Volume 40, pp. 429–435. [Google Scholar]
- Cosier, J.; Glazer, A.M. A nitrogen-gas-stream cryostat for general X-ray diffraction studies. J. Appl. Cryst. 1986, 19, 105–107. [Google Scholar] [CrossRef]
- CrysAlis CCD, CrysAlis RED, CrysAlisPRO; Oxford Diffraction/Agilent Technologies UK Ltd: Yarnton, UK, 2009.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajda-Hermanowicz, K.; Pieniążczak, D.; Zatajska, A.; Wróbel, R.; Drabent, K.; Ciunik, Z. A Study on the Condensation Reaction of 4-Amino-3,5-dimethyl-1,2,4-triazole with Benzaldehydes: Structure and Spectroscopic Properties of Some New Stable Hemiaminals. Molecules 2015, 20, 17109-17131. https://doi.org/10.3390/molecules200917109
Wajda-Hermanowicz K, Pieniążczak D, Zatajska A, Wróbel R, Drabent K, Ciunik Z. A Study on the Condensation Reaction of 4-Amino-3,5-dimethyl-1,2,4-triazole with Benzaldehydes: Structure and Spectroscopic Properties of Some New Stable Hemiaminals. Molecules. 2015; 20(9):17109-17131. https://doi.org/10.3390/molecules200917109
Chicago/Turabian StyleWajda-Hermanowicz, Katarzyna, Damian Pieniążczak, Aleksandra Zatajska, Robert Wróbel, Krzysztof Drabent, and Zbigniew Ciunik. 2015. "A Study on the Condensation Reaction of 4-Amino-3,5-dimethyl-1,2,4-triazole with Benzaldehydes: Structure and Spectroscopic Properties of Some New Stable Hemiaminals" Molecules 20, no. 9: 17109-17131. https://doi.org/10.3390/molecules200917109
APA StyleWajda-Hermanowicz, K., Pieniążczak, D., Zatajska, A., Wróbel, R., Drabent, K., & Ciunik, Z. (2015). A Study on the Condensation Reaction of 4-Amino-3,5-dimethyl-1,2,4-triazole with Benzaldehydes: Structure and Spectroscopic Properties of Some New Stable Hemiaminals. Molecules, 20(9), 17109-17131. https://doi.org/10.3390/molecules200917109