Investigation of Film with β-Galactosidase Designed for Stabilization and Handling in Dry Configuration
Abstract
:1. Introduction
2. Results and Discussion
2.1. Stability of β-Galactosidase in the Films
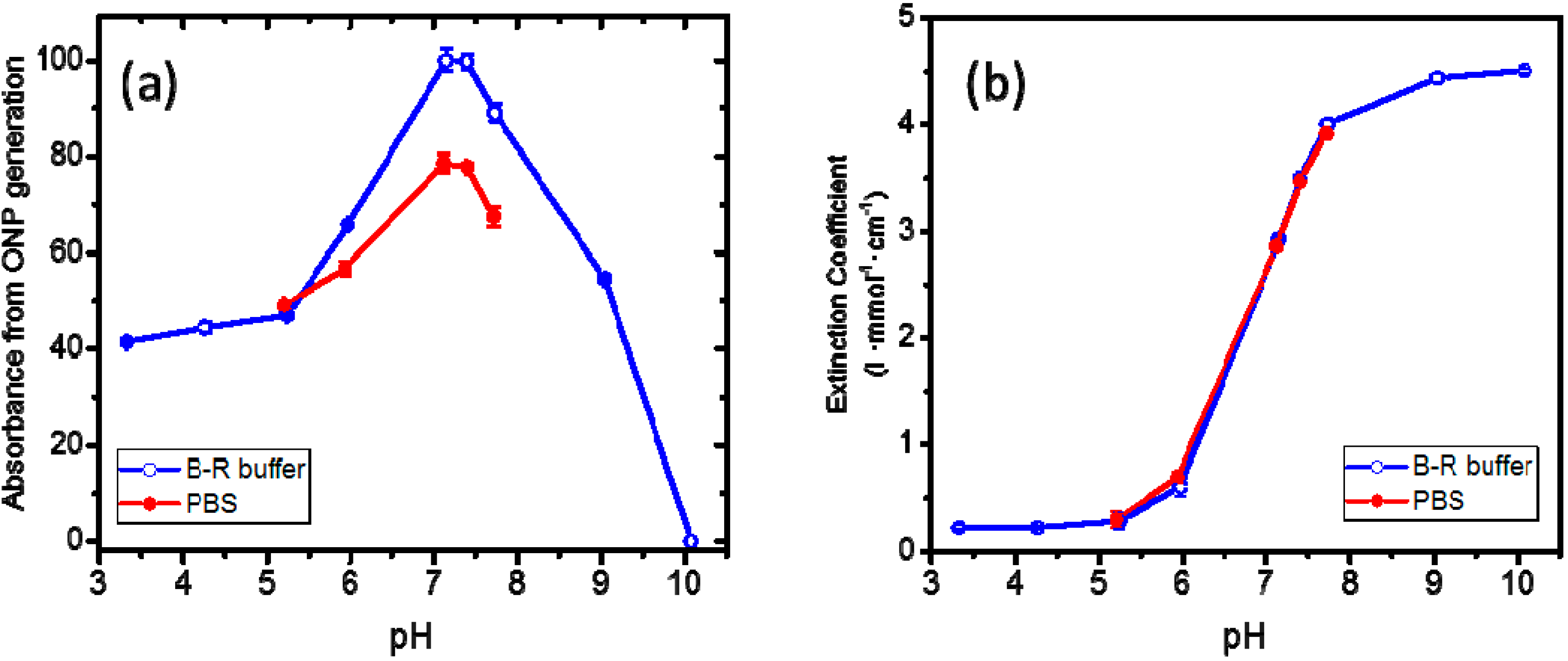




2.2. Mechanical Properties

2.3. Surface Morphology

3. Experimental Section
Material and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frokjaer, S.; Otzen, D.E. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discov. 2005, 4, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kwon, I.C.; Park, K. Oral protein delivery: Current status and future prospect. React. Funct. Polym. 2011, 71, 280–287. [Google Scholar] [CrossRef]
- Morishita, M.; Peppas, N.A. Is the oral route possible for peptide and protein drug delivery? Drug Discov. Today 2006, 11, 905–910. [Google Scholar] [CrossRef] [PubMed]
- De Gonzalo, G.; Orden, A.A.; Bisogno, F.R. New trends in organic synthesis with oxidative enzymes. Curr. Org. Chem. 2012, 16, 2598–2612. [Google Scholar] [CrossRef]
- Schmid, M.; Sängerlaub, S.; Wege, L.; Stäbleret, A. Properties of Transglutaminase Crosslinked Whey Protein Isolate Coatings and Cast Films. Packag. Technol. Sci. 2014, 27, 799–817. [Google Scholar] [CrossRef]
- Sulaiman, S.; Mokhtar, M.N.; Naim, M.N.; Baharuddin, A.S.; Sulaiman, A.A. Review: Potential usage of cellulose nanofibers (CNF) for enzyme immobilization via covalent interactions. Appl. Biochem. Biotechnol. 2015, 175, 1817–1842. [Google Scholar] [PubMed]
- Putney, S.D. Encapsulation of proteins for improved delivery. Curr. Opin. Chem. Biol. 1998, 2, 548–552. [Google Scholar] [CrossRef]
- Putney, S.D.; Burke, P.A. Improving protein therapeutics with sustained-release formulations. Nat. Biotechnol. 1998, 16, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hammann, F.; Schmid, M. Determination and Quantification of Molecular Interactions in Protein Films: A Review. Materials 2014, 12, 7975–7996. [Google Scholar] [CrossRef]
- Manning, M.; Patel, K.; Borchardt, R. Stability of Protein Pharmaceuticals. Pharm. Res. 1989, 6, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int. J. Pharm. 1999, 185, 129–188. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, J.; Aldred, P.; Adhikari, B. Drying and denaturation characteristics of whey protein isolate in the presence of lactose and trehalose. Food Chem. 2014, 177, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Putranto, A.; Aldred, P.; Chen, J.; Adhikari, B. Drying and Denaturation Kinetics of Whey Protein Isolate (WPI) During Convective Air Drying Process. Dry Technol. 2013, 31, 1532–1544. [Google Scholar] [CrossRef]
- Tavakoli-Keshe, R.; Phillips, J.J.; Turner, R.; Bracewell, D.G. Understanding the relationship between biotherapeutic protein stability and solid-liquid interfacial shear in constant region mutants of IgG1 and IgG4. J. Pharm. Sci. 2014, 103, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Tzannis, S.T.; Hrushesky, W.J.M.; Wood, P.A.; Przybycien, T.M. Adsorption of a formulated protein on a drug delivery device surface. J. Colloid Interface Sci. 1997, 189, 216–228. [Google Scholar] [CrossRef]
- Schmid, M.; Reichert, K.; Hammann, F.; Stäbler, A. Storage time-dependent alteration of molecular interaction-property relationships of whey protein isolate-based films and coatings. J. Mater. Sci. 2015, 50, 4396–4404. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Rafael, C.R.; Ángel, B.M.; Roberto, F.L. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar]
- Julia, M.N.; David, T.P.; Anneloes, O.V.; Francisco, J. Immobilization of thermostable β-galactosidase on epoxy support and its use for lactose hydrolysis and galactooligosaccharides. World J. Microbiol. Biotechnol. 2014, 30, 989–998. [Google Scholar]
- Brady, D.; Jordaan, J. Advances in enzyme immobilization. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization-Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Betancor, L.; Luckarift, H.R. Bioinspired enzyme encapsulation for biocatalysis. Trends Biotechnol. 2008, 26, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Sanjust, E. Inorganic materials as supports for covalent enzyme immobilization: Methods and mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Ladero, M.; Garcı́a-Ochoa, F. Kinetic Modeling of Lactose Hydrolysis by a β-Galactosidase from Kluyveromices Fragilis. Enzyme Microb. Technol. 1998, 22, 558–567. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, H.A.; Arreola, S.L.; Mlynek, G.; Djinović-Carugo, K.; Mathiesen, G.; Nguyen, T.H.; Haltrich, D. Homodimeric β-Galactosidase from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081: Expression in Lactobacillus plantarum and Biochemical Characterization. J. Agric. Food Chem. 2012, 60, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Ladero, M.; Santos, A.; Garcı́a, J.L.; Garcı́a-Ochoa, F. Activity over lactose and ONPG of a genetically engineered β-galactosidase from Escherichia coli in solution and immobilized: Kinetic modelling. Enzyme Microb. Technol. 2001, 29, 181–193. [Google Scholar] [CrossRef]
- Fuchsbauer, H.L.; Gerber, U.; Engelmann, J.; Seeger, T.; Sinks, C.; Hecht, T. Influence of gelatin matrices cross-linked with transglutaminase on the properties of an enclosed bioactive material using β-galactosidase as model system. Biomaterials 1996, 17, 1481–1488. [Google Scholar] [CrossRef]
- Kishore, D.; Kayastha, A.M. Optimisation of immobilisation conditions for chick pea β-galactosidase (CpGAL) to alkylamine glass using response surface methodology and its applications in lactose hydrolysis. Food Chem. 2012, 134, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Lipowicz, M.; Basta, N. 2014 Biopharma cold-chain forecast. Pharm. Commer. 2014. Available online: http://pharmaceuticalcommerce.com/index.php?pg=supply_chain_logistics&articleid=27206&keyword=biopharma-cold%20chain-logistics-forecast (accessed on 3 September 2015). [Google Scholar]
- Bishara, R.H. Cold chain management—An essential component of the global pharmaceutical supply chain. Am. Pharm. Rev. 2006, 9, 105–109. [Google Scholar]
- Abdallah, A.A. Global pharmaceutical supply chain: A quality perspective. Int. J. Bus. Manag. 2013, 17, 62–70. [Google Scholar] [CrossRef]
- Mariniello, L.; di Pierro, P.; Esposito, C.; Sorrentino, A.; Masi, P.; Porta, R. Preparation and mechanical properties of edible pectin-soy flour films obtained in the absence or presence of transglutaminase. J. Biotechnol. 2003, 102, 191–198. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocol. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, P.; Huang, J.; Yang, G.; Zheng, L. Ways of strengthening biodegradable soy-dreg plastics. J. Appl. Polym. Sci. 2003, 88, 422–427. [Google Scholar] [CrossRef]
- Anderson, S.L.; Grulke, E.A.; Delassus, P.T.; Smith, P.B.; Kocher, C.W.; Landes, B.G. A Model for Antiplasticization in Polystyrene. Macromolecules 1995, 28, 2944–2954. [Google Scholar] [CrossRef]
- Chamarthy, S.P.; Pinal, R. Moisture induced antiplasticization in microcrystalline cellulose compacts. Tablets Capsul. 2007, 5, 22–33. [Google Scholar]
- Vieira, M.G.A.; da Silva, M.A.; Santos, L.O.D.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Gelin, B.R.; Karplus, M. Side-chain torsional potentials: Effect of dipeptide, proteing and solvent environment. Biochemistry 1979, 18, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Thomazine, M.; Carvalho, R.A.; Sobral, P.J.A. Physical properties of gelatin films plasticized by blends of glycerol and sorbitol. J. Food Sci. 2005, 70, E172–E176. [Google Scholar] [CrossRef]
- Li, N.; Xue, M.H.; Yao, H.; Zhu, J.J. Reagentless biosensor for phenolic compounds based on tyrosinase entrapped within gelatine film. Anal. Bioanal. Chem. 2005, 383, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Wadiak, D.T.; Carbonell, R.G. Kinetic Behavior of Microencapsulated β-Galactosidase. Biotechnol. Bioeng. 1975, 17, 1157–1181. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available. The sample could be obtained as the manuscript describe.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Otte, A.; Xiang, M.; Liu, D.; Pinal, R. Investigation of Film with β-Galactosidase Designed for Stabilization and Handling in Dry Configuration. Molecules 2015, 20, 17180-17193. https://doi.org/10.3390/molecules200917180
Zhang L, Otte A, Xiang M, Liu D, Pinal R. Investigation of Film with β-Galactosidase Designed for Stabilization and Handling in Dry Configuration. Molecules. 2015; 20(9):17180-17193. https://doi.org/10.3390/molecules200917180
Chicago/Turabian StyleZhang, Liguang, Andrew Otte, Min Xiang, Dexiu Liu, and Rodolfo Pinal. 2015. "Investigation of Film with β-Galactosidase Designed for Stabilization and Handling in Dry Configuration" Molecules 20, no. 9: 17180-17193. https://doi.org/10.3390/molecules200917180
APA StyleZhang, L., Otte, A., Xiang, M., Liu, D., & Pinal, R. (2015). Investigation of Film with β-Galactosidase Designed for Stabilization and Handling in Dry Configuration. Molecules, 20(9), 17180-17193. https://doi.org/10.3390/molecules200917180




