Serum Metabolomic Characterization of Liver Fibrosis in Rats and Anti-Fibrotic Effects of Yin-Chen-Hao-Tang
Abstract
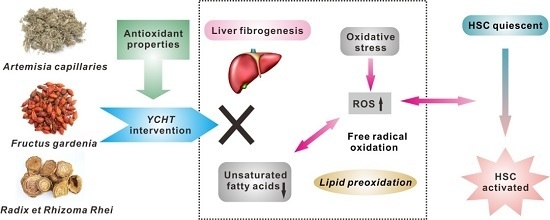
:1. Introduction
2. Results and Discussion
2.1. Histological and Liver Function Examinations

| Parameter | Control | Model | YCHT |
|---|---|---|---|
| Serum | |||
| ALT (U/L) | 30.94 ± 8.07 | 90.07 ± 24.39 ** | 73.8 ± 9.89 ∆∆ |
| AST (U/L) | 55.06 ± 5.23 | 101.43 ± 16.53 ** | 83.3 ± 7.14 ∆∆ |
| Alb (g/L) | 30.48 ± 2.59 | 25.20 ± 2.42 ** | 27.65 ± 2.11 ∆ |
| GGT (U/L) | 95.22 ± 14.12 | 178.26 ± 37.11 ** | 127.39 ± 10.99 ∆∆ |
| ALP (king/100 mL) | 24.97 ± 4.03 | 92.76 ± 28.62 ** | 74.82 ± 13.75 ∆ |
| TBil (mg/dL) | 0.38 ± 0.11 | 0.58 ± 0.11 ** | 0.41 ± 0.06 ∆∆ |
| Liver | |||
| Hydroxyproline (μg/g) | 210.48 ± 65.21 | 463.75 ± 156.72 ** | 307.91 ± 95.06 ∆∆ |
2.2. Metabolic Profiling and Multivariate Analysis


| No. | m/z (amu) | Intraday Repeatability (n = 6) | Interday Repeatability (n = 9) | ||
|---|---|---|---|---|---|
| Mean Peak Area | RSD (%) | Mean Peak Area | RSD (%) | ||
| 1 | 564.3295 | 2847 | 5.0 | 2834 | 6.7 |
| 2 | 588.3300 | 1914 | 5.7 | 1887 | 6.1 |
| 3 | 540.3293 | 5058 | 4.2 | 5055 | 5.2 |
| 4 | 566.3452 | 2664 | 6.6 | 2652 | 7.3 |
| 5 | 568.3609 | 4940 | 3.1 | 4937 | 5.2 |
| 6 | 327.2317 | 1230 | 7.4 | 1194 | 9.8 |
| 7 | 303.2333 | 1541 | 8.5 | 1483 | 10.5 |
| 8 | 279.2325 | 760 | 3.0 | 770 | 8.8 |
| 9 | 281.2478 | 1326 | 8.3 | 1310 | 9.7 |
| No. | VIP Value | tR (min) | Selected Ion | m/z (amu) | Error (ppm) | Formula | Identification Results |
|---|---|---|---|---|---|---|---|
| 1 | 2.86 | 2.54 | [M + HCOO]− | 564.3295 | 1.1 | C26H50NO7P | Lyso-PC C18:2 |
| 2 | 2.41 | 2.58 | [M + HCOO]− | 588.3300 | 0.2 | C28H50NO7P | Lyso-PC C20:4 |
| 3 * | 2.39 | 2.98 | [M + HCOO]− | 540.3293 | 1.5 | C24H50NO7P | Lyso-PC C16:0 |
| 4 | 2.00 | 3.43 | [M + HCOO]− | 566.3452 | 1.1 | C26H52NO7P | Lyso-PC C18:1 |
| 5 | 2.41 | 4.53 | [M + HCOO]− | 568.3609 | 0.9 | C26H54NO7P | Lyso-PC C18:0 |
| 6 * | 2.48 | 6.73 | [M − H]− | 327.2317 | 2.1 | C22H32O2 | Docosahexaenoic acid |
| 7 * | 3.18 | 6.86 | [M − H]− | 303.2333 | 3.0 | C20H32O2 | Arachidonic acid |
| 8 * | 2.18 | 7.03 | [M − H]− | 279.2325 | 0.4 | C18H32O2 | Linoleic acid |
| 9 * | 3.36 | 7.72 | [M − H]− | 281.2478 | 1.1 | C18H34O2 | Oleic acid |


2.3. Fibrosis-Related Variations

2.4. Anti-Fibrotic Effects of YCHT
3. Experimental Section
3.1. Materials and Chemicals
3.2. YCHT Preparation
3.3. Animal Experiment
3.4. Biochemical and Histological Tests
3.5. UPLC-TOF-MS Analysis
3.6. Analytical Method Validation
3.7. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Friedman, S.L. Liver fibrosis-from bench to bedside. J. Hepatol. 2003, 38, S38–S53. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Povero, D.; Busletta, C.; Novo, E.; di Bonzo, L.V.; Cannito, S.; Paternostro, C.; Parola, M. Liver fibrosis: A dynamic and potentially reversible process. Histol. Histopathol. 2010, 25, 1075–1091. [Google Scholar] [PubMed]
- Takahara, Y.; Takahashi, M.; Wagatsuma, H.; Yokoya, F.; Zhang, Q.W.; Yamaguchi, M.; Aburatani, H.; Kawada, N. Gene expression profiles of hepatic cell-type specific marker genes in progression of liver fibrosis. World J. Gastroenterol. 2006, 12, 6473–6499. [Google Scholar] [PubMed]
- Saha, J.K.; Xia, J.; Sandusky, G.E.; Chen, Y.F.; Gerlitz, B.; Grinnell, B.; Jakubowski, J.A. Study of plasma protein C and inflammatory pathways: Biomarkers for dimethylnitrosamine-induced liver fibrosis in rats. Eur. J. Pharmacol. 2007, 575, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.K.; Chung, H.W.; Lee, H.S.; Lim, J.; Park, J.H.; Lim, S.C.; Kim, J.M.; Hong, S.S.; Kwon, S.W. Investigation of metabolite alteration in dimethylnitrosamine-induced liver fibrosis by GC-MS. Bioanalysis 2013, 5, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, J.; Mendoza, F.A.; Jimenez, S.A. Strategies for anti-fibrotic therapies. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.P.; Chen, F.H.; Ling, L.; Dou, P.F.; Bo, H.; Zhong, M.M.; Xia, L.J. Protective effects of total flavonoids of Bidens pilosa L. (TFB) on animal liver injury and liver fibrosis. J. Ethnopharmacol. 2008, 116, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Son, J.Y.; Oh, S.M.; Han, S.H.; Wang, J.H.; Cho, J.H.; Cho, C.K.; Yoo, H.S.; Lee, Y.W.; Lee, M.M. An herbal formula, CGX, exerts hepatotherapeutic effects on dimethylnitrosamine-induced chronic liver injury model in rats. World J. Gastroenterol. 2006, 12, 6142–6148. [Google Scholar] [PubMed]
- Liu, C.; Sun, M.Y.; Wang, L.; Wang, G.Q.; Chen, G.F.; Liu, C.H.; Liu, P. Effects of Yinchenhao Tang and related decoctions on DMN-induced cirrhosis/fibrosis in rats. Chin. Med. 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, M.Y.; Yan, X.F.; Han, L.; Zhang, Y.; Liu, C.H.; El-Nezami, H.; Liu, P. Inhibition of hepatic stellate cell activation following Yinchenhao decoction administration to dimethylnitrosamine-treated rats. Hepatol. Res. 2008, 38, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.L.; Atherton, H.; Shockcor, J.; Atzori, L. Metabolomics as a tool for cardiac research. Nat. Rev. Cardiol. 2011, 8, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.W.; Wang, X.X.; Wu, Y.P.; Lu, B.; Yuan, A.F.; Leon, C.; Guo, N. Urinary metabolomic profiling reveals the effect of Shenfu decoction on chronic heart failure in rats. Molecules 2015, 20, 11915–11929. [Google Scholar] [CrossRef] [PubMed]
- Lindon, J.C.; Nicholson, J.K. Analytical technologies for metabonomics and metabolomics, and multi-omic information recovery. TrAC-Trends Anal. Chem. 2008, 27, 194–204. [Google Scholar] [CrossRef]
- Rainville, P.D.; Theodoridis, G.; Plumb, R.S.; Wilson, I.D. Advances in liquid chromatography coupled to mass spectrometry for metabolic phenotyping. TrAC Trends Anal. Chem. 2014, 61, 181–191. [Google Scholar] [CrossRef]
- Gou, X.J.; Tao, Q.; Feng, Q.; Peng, J.H.; Sun, S.J.; Cao, H.J.; Zheng, N.N.; Zhang, Y.Y.; Hu, Y.Y.; Liu, P. Urinary metabonomics characterization of liver fibrosis induced by CCl4 in rats and intervention effects of Xia Yu Xue Decoction. J. Pharm. Biomed. Anal. 2013, 74, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.J.; Tao, Q.; Feng, Q.; Peng, J.H.; Zhao, Y.; Dai, J.Y.; Wang, W.Y.; Zhang, Y.Y.; Hu, Y.Y.; Liu, P. Urine metabolic profile changes of CCl4-liver fibrosis in rats and intervention effects of Yi Guan Jian Decoction using metabonomic approach. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tao, Q.; Sun, M.Y.; Wu, J.Z.; Yang, W.G.; Jian, P.; Peng, J.H.; Hu, Y.Y.; Liu, C.H.; Liu, P. Kupffer cells are associated with apoptosis, inflammation and fibrotic effects in hepatic fibrosis in rats. Lab. Investig. 2010, 90, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Wang, J.R.; Yau, L.F.; Ho, H.M.; Chan, C.L.; Hu, P.; Liu, L.; Jiang, Z.H. A cellular lipidomic study on the Aβ-induced neurotoxicity and neuroprotective effects of EGCG by using UPLC/MS-based glycerolipids profiling and multivariate analysis. Mol. Biosyst. 2012, 8, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Chen, X.; Hu, P.; Liang, Q.L.; Liang, X.P.; Wang, Y.M.; Luo, G.A. Metabolomic profiling of rat serum associated with isoproterenol-induced myocardial infarction using ultra-performance liquid chromatography/time-of-flight mass spectrometry and multivariate analysis. Talanta 2009, 79, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Hepatic fibrosis-Overview. Toxicology 2008, 254, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zern, M.A. Hepatic stellate cells: A target for the treatment of liver fibrosis. J. Gastroenterol. 2000, 35, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Poli, G. Pathogenesis of liver fibrosis: Role of oxidative stress. Mol. Asp. Med. 2000, 21, 49–98. [Google Scholar] [CrossRef]
- Broza, C.; Muntean, D.; Dehelean, C.; Savoiu, G.; Serban, C.; Simu, G.; Andoni, M.; Butur, M.; Dragan, S. Oxidative stress and lipid peroxidation-A lipid metabolism dysfunction. In Lipid Metabolism; Baez, R.V., Ed.; InTech Publisher: Rijeka, Croatia, 2013; pp. 23–38. [Google Scholar]
- Shimizu, I.; Shimamoto, N.; Saiki, K.; Furujo, M.; Osawa, K. Lipid peroxidation in hepatic fibrosis. In Lipid Peroxidation; Catala, A., Ed.; InTech Publisher: Rijeka, Croatia, 2012; pp. 483–492. [Google Scholar]
- Pamplona, R.; Portero-Otin, M.; Riba, D.; Requena, J.R.; Thorpe, S.R.; Lopez-Torres, M.; Barja, G. Low fatty acid unsaturation: A mechanism for lowered lipoperoxidative modification of tissue proteins in mammalian species with long life spans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, B286–B291. [Google Scholar] [CrossRef]
- Angelico, M.; Alvaro, D.; Cantafora, A.; Masella, R.; Gaudio, E.; Gandin, C.; Ginanni-Corradini, S.; Ariosto, F.; Riggio, O.; Capocaccia, L. Impaired hepatic handling and processing of lysophosphatidylcholine in rats with liver cirrhosis. Gastroenterology 1991, 101, 228–237. [Google Scholar] [PubMed]
- Hishikawa, D.; Shindou, H.; Kobayashi, S.; Nakanishi, H.; Taguchi, R.; Shimizu, T. Discovery essential of a lysophospholipid acyltransferase family for membrane asymmetry and diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 2830–2835. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Sun, W.J.; Sun, H.; Lv, H.T.; Wu, Z.M.; Wang, P.; Liu, L.; Cao, H.X. Analysis of the constituents in the rat plasma after oral administration of Yin Chen Hao Tang by UPLC/Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2008, 46, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Saile, B.; Ramadori, G. Inflammation, damage repair and liver fibrosis-Role of cytokines and different cell types. Z. Gastroenterol. 2007, 45, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.T.; Dong, L.; Dang, X.Y.; Liu, Y.P.; Jiang, J.; Wang, Y.; Lu, X.L.; Guo, X.Y. Effect of chlorogenic acid on LPS-induced proinflammatory signaling in hepatic stellate cells. Inflamm. Res. 2013, 62, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Machida, K.; Onodera, R.; Furuta, K.; Kikuchi, M. Studies of the constituents of Gardenia species. I. Monoterpenoids from Gardeniae Fructus. Chem. Pharm. Bull. 1998, 46, 1295–1300. [Google Scholar] [CrossRef]
- Ma, T.T.; Huang, C.; Zong, G.J.; Zha, D.J.; Meng, X.M.; Li, J.; Tang, W.J. Hepatoprotective effects of geniposide in a rat model of nonalcoholic steatohepatitis. J. Pharm. Pharmacol. 2011, 63, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Inao, M.; Mochida, S.; Matsui, A.; Eguchi, Y.; Yulutuz, Y.; Wang, Y.H.; Naiki, K.; Kakinuma, T.; Fujimori, K.; Nagoshi, S.; Fujiwara, K. Japanese herbal medicine Inchin-ko-to as a therapeutic drug for liver fibrosis. J. Hepatol. 2004, 41, 584–591. [Google Scholar] [CrossRef] [PubMed]
- El-Beshbishy, H.A.; Hassan, M.H.; Aly, H.A.A.; Doghish, A.S.; Alghaithy, A.A.A. Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol. Environ. Saf. 2012, 83, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.S.; Ho, H.H.; Lin, M.C.; Chyau, C.C.; Peng, J.S.; Wang, C.J. Mulberry water extracts (MWEs) ameliorated carbon tetrachloride-induced liver damages in rat. Food Chem. Toxicol. 2012, 50, 3086–3093. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Wu, J.H.; Huang, C.C.; Peng, H.C.; Chen, Y.L.; Yang, S.C.; Chang, S.T. Protective effect of Acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food Chem. Toxicol. 2009, 47, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, L.; White, J.; Fang, J. Inhibitory effect of gallic acid on CCl4-mediated liver fibrosis in mice. Cell Biochem. Biophys. 2014, 69, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Wu, G.; Park, H.J.; Jiang, P.P.; Sit, W.H.; van Griensven, L.J.L.D.; Wan, J.M.F. Protective effect of Phellinus linteus polysaccharide extracts against thioacetamide-induced liver fibrosis in rats: A proteomics analysis. Chin. Med. 2012, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamall, I.S.; Finelli, V.N.; Que Hee, S.S. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal. Biochem. 1981, 112, 70–75. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds used in the study are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, X.; Hu, P.; Zhou, W.; Zhang, M.; Liu, J.; Wang, Y.; Liu, P.; Luo, G. Serum Metabolomic Characterization of Liver Fibrosis in Rats and Anti-Fibrotic Effects of Yin-Chen-Hao-Tang. Molecules 2016, 21, 126. https://doi.org/10.3390/molecules21010126
Zhang H, Wang X, Hu P, Zhou W, Zhang M, Liu J, Wang Y, Liu P, Luo G. Serum Metabolomic Characterization of Liver Fibrosis in Rats and Anti-Fibrotic Effects of Yin-Chen-Hao-Tang. Molecules. 2016; 21(1):126. https://doi.org/10.3390/molecules21010126
Chicago/Turabian StyleZhang, Hongyang, Xiaoning Wang, Ping Hu, Wenjun Zhou, Min Zhang, Jia Liu, Yuerong Wang, Ping Liu, and Guoan Luo. 2016. "Serum Metabolomic Characterization of Liver Fibrosis in Rats and Anti-Fibrotic Effects of Yin-Chen-Hao-Tang" Molecules 21, no. 1: 126. https://doi.org/10.3390/molecules21010126
APA StyleZhang, H., Wang, X., Hu, P., Zhou, W., Zhang, M., Liu, J., Wang, Y., Liu, P., & Luo, G. (2016). Serum Metabolomic Characterization of Liver Fibrosis in Rats and Anti-Fibrotic Effects of Yin-Chen-Hao-Tang. Molecules, 21(1), 126. https://doi.org/10.3390/molecules21010126