3.2.3. General Procedure for the Synthesis of Imidazo[1,2-a]pyridine amide-cinnamamide Hybrids 11–14
A mixture of imidazo[1,2-a]pyridine-3-carboxylic acids 9, 10 (1.0 mmol) and BOP-Cl (1.2 mmol) in dry DCM (10 mL) was stirred under N2 at room temperature for 5 min at room temperature. Then, Et3N (2.2 mmol) was added followed by 5a–l and 6a–g (1.2 mmol). The resulting suspension was allowed to continue stirring for 3 h. DCM (10 mL) was added and washed with 0.2 M HCl (20 mL), H2O (20 mL), 5% satd. NaHCO3 (20 mL) and brine (20 mL), then dried over anhydrous magnesium sulfate. The solvent was removed in vacuo. Silica flash column chromatography eluting with DCM/MeOH (95:5) yielded 11–14.
(E)-2,6-Dimethyl-N-(2-(3-p-tolylacrylamido)ethyl)imidazo[1,2-a]pyridine-3-carboxamide (11a): The title compound was prepared from 5a and 9 as a white solid (61%); m.p.: 215–217 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.82 (s, 1H, pyridine-H), 8.27 (d, J = 5.5 Hz, 1H, -CONH-), 7.86 (d, J = 5.0 Hz, 1H, -CONH-), 7.47–7.44 (m, 3H, Ar-H), 7.40 (d, J = 16.0 Hz, 1H, =C-H), 7.23–7.20 (m, 3H, Ar-H), 6.58 (d, J = 16.0 Hz, 1H, =C-H), 3.44–3.41 (m, 4H, 2 × -CH2-), 2.54 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.28 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.98, 161.57, 145.33, 144.25, 139.68, 139.14, 132.57, 130.00, 129.63, 127.96, 125.22, 122.39, 121.53, 116.14, 115.92, 38.96, 21.41, 18.24, 16.07. MS-ESI (m/z): 377 [M + H]+.
(E)-2,6-Dimethyl-N-(2-(3-(3,4,5-trimethoxyphenyl)acrylamido)ethyl)imidazo[1,2-a]pyridine-3-carboxamide (11b): The title compound was obtained from 5b and 9 as a white solid (63%)’ m.p.: 224–226 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.83 (s, 1H, pyridine-H), 8.24 (t, J = 5.0 Hz, 1H, -CONH-), 7.85 (t, J = 5.0 Hz, 1H, -CONH-), 7.47 (d, J = 9.0 Hz, 1H, pyridine-H), 7.38 (d, J = 15.5 Hz, 1H, =C-H), 7.23 (dd, J = 9.0, 1.5 Hz, 1H, pyridine-H), 6.90 (s, 2H, Ar-H), 6.60 (d, J = 15.5 Hz, 1H, =C-H), 3.80 (s, 6H, -OCH3), 3.67 (s, 3H, CH3), 3.44–3.41 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.28 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.48, 161.13, 153.08, 144.90, 143.82, 138.93, 138.60, 130.50, 129.18, 124.78, 121.95, 121.45, 115.68, 115.49, 104.91, 60.10, 55.87, 54.96, 38.58, 30.99, 17.79, 15.66. MS-ESI (m/z): 453 [M + H]+.
(E)-N-(2-(3-(3,4-Dichlorophenyl)acrylamido)ethyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (11c): The title compound was prepared from 5c and 9 as a white solid (57%); m.p.: 213–215 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.82 (s, 1H, pyridine-H), 8.37 (m, 1H, -CONH-), 7.88–7.83 (m, 2H, -CONH- and Ar-H), 6.65 (d, J = 8.5 Hz, 1H, Ar-H), 7.55 (dd, J = 8.0, 1.5 Hz, 1H, Ar-H), 7.46 (d, J = 9.0 Hz, 1H, pyridine-H), 7.42 (d, J = 16.0 Hz, 1H, =C-H), 7.22 (dd, J = 9.5, 2.0 Hz, 1H, Ar-H), 6.72 (d, J = 16.0 Hz, 1H, =C-H), 3.44 ( m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.27 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 164.94, 161.16, 144.92, 143.82, 136.16, 135.84, 131.69, 131.61, 131.06, 129.44, 129.17, 127.28, 124.78, 124.44, 121.94, 115.67, 115.46, 38.86, 38.72, 17.79, 15.66. MS-ESI (m/z): 431 [M + H]+.
(E)-N-(2-(3-(4-Methoxyphenyl)acrylamido)ethyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (11d): The title compound was prepared from 5d and 9 as a white solid (69%); m.p.: 203–205 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.12 (s, 1H, pyridine-H), 7.55 (d, J = 15.5 Hz, 1H, =C-H), 7.44 (d, J = 9.0 Hz, 1H, pyridine-H), 7.40 (d, J = 9.0 Hz, 1H, pyridine-H), 7.14 (dd, J = 9.0, 1.5 Hz, 1H, Ar-H), 6.85 (d, J = 9.0 Hz, 1H, Ar-H), 6.80 (s, 1H, -CONH-), 6.60 (s, 1H, -CONH-), 6.29 (d, J = 15.5 Hz, 1H, =C-H), 3.80 (s, 3H, OCH3), 3.69–3.67 (m, 4H, 2 × -CH2-), 2.71 (s, 3H, CH3), 2.31 (s, 3H, CH3). 13C-NMR (126 MHz, CDCl3) δ (ppm): 167.73, 162.64, 161.11, 145.81, 144.99, 145.37, 130.11, 129.55, 127.38, 125.96, 123.10, 117.73, 115.71, 115.21, 114.35, 55.48, 41.06, 40.12, 18.49, 16.59. MS-ESI (m/z): 393 [M + H]+.
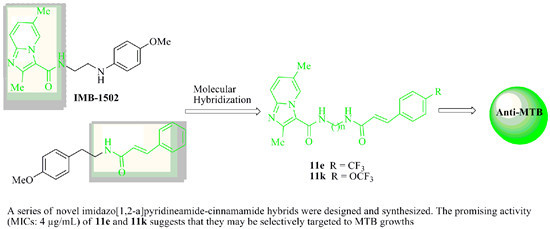
(E)-2,6-Dimethyl-N-(2-(3-(4-(trifluoromethyl)phenyl)acrylamido)ethyl)imidazo[1,2-a]pyridine-3-carboxamide (11e): The title compound was prepared from 5e and 9 as a white solid (72%); m.p.: 252–254 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.82 (s, 1H, pyridine-H), 8.82–8.40 (m, 1H, Ar-H), 7.87–7.85 (m, 1H, Ar-H), 7.77 (dd, J = 9.0, 11.5 Hz, 1H, Ar-H), 7.52 (d, J = 16.0 Hz, 1H, =C-H), 7.46 (d, J = 9.0 Hz, 1H, pyridine-H), 7.22 (dd, J = 9.0, 1.5 Hz, 1H, Ar-H), 6.77 (d, J = 16.0 Hz, 1H, =C-H), 3.46–3.43 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.26 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 164.91, 161.16, 144.92, 143.83, 138.98, 137.08, 129.33, 129.17, 128.17, 125.84, 125.81, 125.21, 124.95, 124.76, 121.93, 115.68, 115.49, 38.87, 38.62, 17.78, 15.65. HRMS-ESI (m/z): calcd. for C22H22O2N4F3 [M + H]+: 431.1695; found 431.1675.
(E)-N-(2-(3-(2-Fluorophenyl)acrylamido)ethyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (11f): The title compound was prepared from 5f and 9 as a white solid (70%); m.p.: 197–199 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.82 (s, 1H, pyridine-H), 8.42 (t, J = 5.0 Hz, 1H, -CONH-), 7.86 (t, J = 5.0 Hz, 1H, -CONH-), 7.67–7.64 (m, 1H, Ar-H), 7.52–7.40 (m, 3H, =C-H and Ar-H), 7.30–7.21 (m, 3H, pyridine-H and Ar-H), 6.74 (d, J = 16.0 Hz, 1H, =C-H), 3.45–3.42 (m, 4H, 2 × -CH2-), 2.54 (s, 3H, CH3), 2.28 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.13, 161.48, 161.15, 159.49, 144.90, 143.82, 131.37, 131.30, 131.16, 129.22, 129.20, 129.18, 125.05, 124.99, 124.77, 121.94, 116.21, 116.04, 115.71, 115.49, 38.87, 38.60, 17.77, 15.62. MS-ESI (m/z): 381 [M + H]+.
(E)-N-(2-(3-(4-Fluorophenyl)acrylamido)ethyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (11g): The title compound was prepared from 5g and 9 as a white solid (67%); m.p.: 208–211 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 9.14 (s, 1H, pyridine-H), 7.55 (d, J = 15.5 Hz, 1H, =C-H), 7.46–7.42 (m, 3H, pyridine-H and Ar-H), 7.04 (dd, J = 9.0, 1.5 Hz, 1H, Ar-H), 7.05–7.01 (m, 2H, Ar-H), 6.67 (s, 1H, -CONH-), 6.60 (s, 1H, -CONH-), 6.34 (d, J = 15.5 Hz, 1H, =C-H), 3.72–3.67 (m, 4H, 2 × -CH2-), 2.72 (s, 3H, CH3), 2.32 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 162.12, 159.72, 157.81, 157.73, 140.98, 140.17, 135.50, 125.94, 125.92, 125.11, 124.81, 124.74, 120.97, 118.11, 114.94, 114.92, 111.16, 110.99, 110.84, 110.09, 35.81, 35.41, 13.52, 11.74. MS-ESI (m/z): 381 [M + H]+.
(E)-N-(2-(3-(3-Fluorophenyl)acrylamido)ethyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (11h): The title compound was prepared from 5h and 9 as a white solid (65%); m.p.: 172–175 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.83 (s, 1H, pyridine-H), 8.32 (d, J = 5.5 Hz, 1H, -CONH-), 7.83 (d, J = 5.5 Hz, 1H, -CONH-), 7.46–7.39 (m, 5H, Ar-H), 7.22–7.19 (m, 2H, Ar-H and pyridine-H), 6.34 (d, J = 15.5 Hz, 1H, =C-H), 3.47–3.36 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.27 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.12, 163.45, 161.51, 161.18, 144.93, 143.84, 137, 55, 137.49, 130.96, 130.89, 129.17, 124, 78, 123.71, 121.95, 116.24, 116.07, 115.69, 115.49, 114.05, 113.88, 38.91, 38.62, 17.78, 15.65. MS-ESI (m/z): 381 [M + H]+.
(E)-N-(2-(3-(4-Chlorophenyl)acrylamido)ethyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (11i): The title compound was prepared from 5i and 9 as a white solid (59%); m.p.: 252–254 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.82 (s, 1H, pyridine-H), 8.31 (s, 1H, -CONH-), 7.83 (s, 1H, -CONH-), 7.59 (d, J = 8.5 Hz, 2H, Ar-H), 7.48–7.40 (m, 4H, Ar-H and pyridine-H), 7.23 (dd, J = 9.0, 1.5 Hz, 1H, Ar-H), 6.64 (d, J = 16.0 Hz, 1H, =C-H), 3.45–3.41 (m, 4H, 2 × -CH2-), 2.54 (s, 3H, CH3), 2.28 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.19, 161.15, 144.91, 143.82, 137.40, 133.90, 133.86, 129.25, 129.18, 128.99, 124.77, 122.95, 121.95, 115.69, 115.48, 38.93, 38.57, 17.79, 15.64. MS-ESI (m/z): 397 [M + H]+.
(E)-2,6-Dimethyl-N-(2-(3-(4-nitrophenyl)acrylamido)ethyl)imidazo[1,2-a]pyridine-3-carboxamide (11J): The title compound was prepared from 5J and 9 as a white solid (52%); m.p.: 213–215 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.82 (s, 1H, pyridine-H), 8.48 (s, 1H, -CONH-), 8.24 (d, J = 9.0 Hz, 2H, pyridine-H), 7.87–7.82 (m, 3H, -CONH- and Ar-H), 7.55 (d, J =15.5 Hz, 1H, =C-H), 7.45 (d, J = 9.0 Hz, 2H, pyridine-H) 7.21 (dd, J = 9.0, 1.5 Hz, 1H, Ar-H), 6.84 (d, J = 16.0 Hz, 1H, =C-H), 3.47–3.43 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.27 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 164.70, 161.16, 147.49, 144.94, 143.83, 141.51, 136.43, 129.16, 128.59, 126.43, 124.76, 124.13, 121.94, 115.66, 115.48, 38.82, 38.68, 17.80, 15.66. MS-ESI (m/z): 408 [M + H]+.
(E)-2,6-Dimethyl-N-(2-(3-(4-(trifluoromethoxy)phenyl)acrylamido)ethyl)imidazo[1,2-a]pyridine-3-carboxamide (11k): The title compound was prepared from 5k and 9 as a white solid (64%); m.p.: 214–217 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.82 (s, 1H, pyridine-H), 8.81–8.42 (m, 1H, -CONH-), 7.85–7.82 (m, 1H, -CONH-), 7.70 (dd, J = 9.0, 11.5 Hz, 1H, Ar-H), 7.58 (d, J = 16.0 Hz, 1H, =C-H), 7.48 (d, J = 9.0 Hz, 1H, pyridine-H), 7.23 (dd, J = 9.0, 1.5 Hz, 1H, Ar-H), 6.97 (d, J = 16.0 Hz, 1H, =C-H), 3.50–3.45 (m, 4H, 2 × -CH2-), 2.58 (s, 3H, CH3), 2.24 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.90, 162.15, 145.82, 143.83, 139.92, 137.18, 129.32, 129.17, 128.18, 126.85, 125.98, 125.31, 124.85, 124.74, 122.43, 114.90, 114.49, 38.85, 37.65, 18.75, 15.65. HRMS-ESI (m/z): calcd. for C22H22O3N4F3 [M + H]+: 447.1644; found 447. 1622.
(E)-N-(2-Cinnamamidoethyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (11l): The title compound was obtained from 5l and 9 as a white solid (58%); m.p.: 196–198 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.22 (s, 1H, pyridine-H), 7.65 (d, J = 16.0 Hz, 1H, =C-H), 7.52–7.47 (m, 3H, Ar-H and pyridine-H), 7.38–7.36 (m, 3H, Ar-H and pyridine-H), 7.18 (dd, J = 9.0, 2.0 Hz, 1H, Ar-H), 6.89 (t, J = 6.0 Hz, 1H, -CONH-), 6.58 (t, J = 6.0 Hz, 1H, -CONH-), 6.48 (d, J = 16.0 Hz, 1H, =C-H), 3.59 (dd, J = 12.0, 6.5 Hz, 2H, -CH2-), 3.54 (dd, J = 12.0, 6.5 Hz, 2H, -CH2-), 2.83 (s, 3H, CH3), 2.37 (s, 3H, CH3). 13C-NMR (126 MHz, CDCl3) δ (ppm): 166.96, 162.28, 145.66, 145.03, 141.40, 134.81, 129.97, 129.88, 128.94, 127.93, 126.04, 123.00, 120.55, 115.76, 115.39, 36.26, 35.66, 30.53, 18.52, 16.79. MS-ESI (m/z): 363 [M + H]+.
(E)-2,7-Dimethyl-N-(2-(3-p-tolylacrylamido)ethyl)imidazo[1,2-a]pyridine-3-carboxamide (12a): The title compound was prepared from 5a and 10 as a white solid (61%); m.p.: 244–247 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.94 (d, J = 7.5 Hz, 1H, pyridine-H), 8.28 (d, J = 5.0 Hz, 1H, -CONH-), 7.79 (d, J = 5.0 Hz, 1H, -CONH-), 7.45 (d, J = 8.0 Hz, 2H, Ar-H), 7.40 (d, J = 15.5 Hz, 1H, =C-H), 7.34–7.33 (m, 1H, Ar-H and pyridine-H), 7.22 (d, J = 8.0 Hz, 2H, Ar-H), 6.58 (d, J = 15.5 Hz, 1H, =C-H), 3.45–3.41 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.36 (s, 3H, CH3), 2.32 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.55, 161.15, 145.27, 145.16, 139.23, 138.71, 137.12, 132.12, 129.55, 127.52, 126.43, 121.08, 115.33, 115.15, 114.54, 38.52, 20.96, 20.73, 15.71. MS-ESI (m/z): 377 [M + H]+.
(E)-2,7-Dimethyl-N-(2-(3-(3,4,5-trimethoxyphenyl)acrylamido)ethyl)imidazo[1,2-a]pyridine-3-carboxamide (12b): The title compound was obtained from 5b and 10 as a white solid (61%); m.p.: 225–227 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.92 (d, J = 7.0 Hz, 1H, pyridine-H), 8.24 (t, J = 5.0 Hz, 1H, -CONH-), 7.85 (t, J = 5.0 Hz, 1H, -CONH-), 7.47 (d, J = 9.0 Hz, 1H, pyridine-H), 7.38 (d, J = 15.5 Hz, 1H, =C-H), 7.23 (dd, J = 7.0, 1.5 Hz, 1H, Ar-H), 6.90 (s, 2H, Ar-H), 6.60 (d, J = 15.5 Hz, 1H, =C-H), 3.80 (s, 6H, 2 × OCH3), 3.67 (s, 3H, OCH3), 3.44–3.41 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.28 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 166.38, 162.14, 154.10, 145.12, 143.83, 138.94, 138.50, 131.40, 128.19, 124.68, 122.14, 121.45, 115.67, 115.40, 105.81, 61.15, 56.88, 53.97, 39.57, 31.87, 18.80, 15.64. MS-ESI (m/z): 453 [M + H]+.
(E)-N-(2-(3-(3,4-Dichlorophenyl)acrylamido)ethyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (12c): The title compound was prepared from 5c and 10 as a white solid (54%); m.p.: 201–204 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.92 (d, J = 7.5 Hz, 1H, pyridine-H), 8.34 (d, J = 5.5 Hz, 1H, -CONH-), 7.84 (d, J = 2.0 Hz, 1H, pyridine-H), 7.78 (d, J = 5.0 Hz, 1H, -CONH-), 7.66 (d, J = 8.0, 1H, pyridine-H), 7.55 (dd, J = 8.5, 2.0 Hz, 1H, Ar-H), 7.42 (d, J = 16.0 Hz, 1H, =C-H), 7.32 (s, 1H, Ar-H), 6.82 (dd, J = 7.5, 2.0 Hz, 1H, Ar-H), 6.70 (d, J = 16.0 Hz, 1H, =C-H), 3.43 ( m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.35 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 164.93, 161.16, 145.26, 145.15, 137.15, 136.19, 135.84, 131.69, 131.61, 131.07, 129.48, 127.28, 126.44, 124.42, 115.32, 115.16, 114.53, 38.87, 38.63, 20,72, 15.72. MS-ESI (m/z): 431 [M + H]+.
(E)-N-(2-(3-(4-Methoxyphenyl)acrylamido)ethyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (12d): The title compound was prepared from 5d and 10 as a white solid (67%); m.p.: 218–221 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.94 (d, J = 7.0 Hz, 1H, pyridine-H), 8.21 (d, J = 5.0 Hz, 1H, -CONH-), 7.78 (d, J = 5.0 Hz, 1H, -CONH-), 7.79–7.77 (m, 2H, Ar-H and pyridine-H), 7.34–7.37 (m, 2H, Ar-H and pyridine-H), 6.98–6.95 (m, 2H, Ar-H), 6.86–6.84 (m, 2H, Ar-H), 6.48 (d, J = 16.0 Hz, 1H, =C-H), 3.78 (s, 3H, OCH3), 3.44–3.40 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.36 (s, 3H, CH3). 13C-NMR (126 MHz, CDCl3) δ (ppm): 165.72, 161.13, 160.34, 145.27, 145.15, 138.47, 137.13, 129.13, 127.43, 126.44, 119.62, 115.34, 115.16, 114.55, 114.40, 55.27, 38.49, 20.73, 15.70. MS-ESI (m/z): 393 [M + H]+.
(E)-2,7-Dimethyl-N-(2-(3-(4-(trifluoromethyl)phenyl)acrylamido)ethyl)imidazo[1,2-a]pyridine-3-carboxamide (12e): The title compound was prepared from 5e and 10 as a white solid (72%); m.p.: 218–221 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.83 (s, 1H, pyridine-H), 8.38 (t, J = 5.0, 1H, -CONH-), 7.83 (t, J = 5.0, 1H, -CONH-) 7.79–7.75 (m, 4H, Ar-H and pyridine-H), 7.51 (d, J = 16.0 Hz, 1H), 7.46 (d, J = 9.0 Hz, 1H, Ar-H), 7.22 (dd, J = 9.0, 1.5 Hz, 1H, Ar-H), 6.77 (d, J = 16.0 Hz, 1H, =C-H), 3.46–3.43 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.27 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.71, 161.13, 160.34, 145.27, 145.15, 138.47, 137.13, 129.13, 127.43, 126.44, 119.62, 115.34, 115.16, 114.54, 114.40, 55.27, 38.49, 20.73, 15.70. MS-ESI (m/z): 431 [M + H]+.
(E)-N-(2-(3-(2-Fluorophenyl)acrylamido)ethyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (12f): The title compound was prepared from 5f and 10 as a white solid (61%); m.p.: 213–216 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.92 (d, J = 7.0 Hz, 1H, pyridine-H), 8.44 (d, J = 5.0 Hz, 1H, -CONH-), 7.79 (d, J = 5.0 Hz, 1H, -CONH-), 7.67–7.63 (m, 1H, Ar-H), 7.51 (d, J = 16.0 Hz, 1H, =C-H), 7.43–7.40 (m, 1H, Ar-H), 7.32 (s, 1H), 7.29–7.23 (m, 2H, Ar-H), 6.81 (dd, J = 7.5, 1.5 Hz, 1H), 6.74 (d, J = 16.0 Hz, 1H, =C-H), 3.45–3.42 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.35 (s, 3H, CH3). 13C-NMR (126 MHz, CDCl3) δ (ppm): 165.59, 161.92, 161.60, 159.93, 145.72, 145.60, 137.56, 131.79, 131.61, 129.65, 129.63, 126.87, 125.47, 125.44, 125.42, 116.64, 116.47, 115.78, 115.57, 114.98, 39.34, 39.05, 21.16, 16.14. MS-ESI (m/z): 381 [M + H]+.
(E)-N-(2-(3-(4-Fluorophenyl)acrylamido)ethyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (12g): The title compound was prepared from 5g and 10 as a white solid (65%); m.p.: 246–248 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.93 (d, J = 7.5 Hz, 1H, pyridine-H), 8.30 (t, J = 5.0 Hz, 1H, -CONH-), 7.62 (t, J = 3.5 Hz, 1H, -CONH-), 7.64–7.61 (m, 1H, Ar-H), 7.43 (d, J = 16.0 Hz, 1H, =C-H), 7.33 (s, 1H, Ar-H), 7.27–7.23 (m, 2H, Ar-H and pyridine-H), 6.83 (dd, J = 7.0, 1.5 Hz, 1H), 6.57 (d, J = 16.0 Hz, 1H, =C-H), 3.44–3.40 (m, 4H, 2 × -CH2-), 2.54 (s, 3H, CH3), 2.36 (s, 3H, CH3). 13C-NMR (126 MHz, CDCl3) δ (ppm): 165.77, 164.13, 162.17, 161.59, 145.71, 145.59, 138.02, 137.60, 131.98, 131.95, 130.19, 130.12, 126.88, 122.47, 116.47, 116.30, 115.78, 115.62, 114.98, 39.41, 38.99, 21.18, 16.15. MS-ESI (m/z): 381 [M + H]+.
(E)-N-(2-(3-(3-Fluorophenyl)acrylamido)ethyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (12h): The title compound was prepared from 5h and 10 as a white solid (50%); m.p.: 172–175 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.93 (d, J = 7.5 Hz, 1H, pyridine-H), 8.33 (t, J = 5.0 Hz, 1H, -CONH-), 7.78 (t, J = 5.0 Hz, 1H, -CONH-), 7.48-7.40 (m, 4H, Ar-H and pyridine-H), 7.33 (s, 1H, Ar-H), 7.23–7.18 (m, 1H, Ar-H), 6.83 (dd, J = 7.0, 1.5 Hz, 1H, Ar-H), 6.58 (d, J = 16.0 Hz, 1H, =C-H), 3.44–3.41 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.36 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.10, 163.44, 161.51, 161.16, 145.28, 145.17, 137.55, 137.48, 137.14, 130.96, 130.89, 126.44, 123.70, 116.24, 116.07, 115.33, 115.16, 114.55, 114.06, 113.89, 38.91, 38.59, 20.73, 15.71. MS-ESI (m/z): 381 [M + H]+.
(E)-N-(2-(3-(4-Chlorophenyl)acrylamido)ethyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (12i): The title compound was prepared from 5i and 10 as a white solid (61%); m.p.: 172–175 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.83 (s, 1H, pyridine-H), 8.32 (d, J = 5.5 Hz, 1H, -CONH-), 7.83 (d, J = 5.0 Hz, 1H, -CONH-), 7.46–7.39 (m, 5H, pyridine-H, Ar-H and =C-H), 7.21 (d, J = 9.0 Hz, 1H, Ar-H), 6.69 (d, J = 15.5 Hz, 1H, =C-H, Ar-H), 3.47–3.43 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.27 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.56, 161.60, 149.25, 145.72, 145.61, 137.59, 134.72, 129.89, 129.79, 126.88, 123.80, 121.91, 121.51, 119,47, 115.77, 115.61, 114.99, 39.36, 39.01, 31.17, 13.15. MS-ESI (m/z): 397 [M + H]+.
(E)-2,7-Dimethyl-N-(2-(3-(4-nitrophenyl)acrylamido)ethyl)imidazo[1,2-a]pyridine-3-carboxamide (11j): The title compound was prepared from 5j and 10 as a white solid (71%); m.p.: 213–215 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.93 (d, J = 7.0 Hz, 1H, pyridine-H), 8.45 (s, 1H, -CONH-), 8.25 (d, J = 8.5 Hz, 1H, Ar-H), 7.83 (d, J = 8.5 Hz, 1H, Ar-H), 7.78 (d, J = 5.5 Hz, 1H, -CONH-), 7.55 (d, J = 16.0 Hz, 1H, =C-H, Ar-H), 7.33 (s, 1H, Ar-H), 6.85–6.80 (m, 2H, Ar-H), 3.44 (s, 4H, 2 × -CH2-), 2.54 (s, 3H, CH3), 2.36 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 164.69, 161.17, 147.52, 145.28, 145.18, 141.51, 137.15, 136.46, 128.62, 126.43, 124.16, 115.31, 115.17, 114.55, 38.83, 38.66, 20.73, 15.72. MS-ESI (m/z): 408 [M + H]+.
(E)-2,6-Dimethyl-N-(2-(3-(4-(trifluoromethoxy)phenyl)acrylamido)ethyl)imidazo[1,2-a]pyridine-3-carboxamide (12k): The title compound was prepared from 5k and 10 as a white solid (61%); m.p.: 214–216 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.93 (d, J = 7.0 Hz, 1H, pyridine-H), 8.33 (d, J = 5.0 Hz, 1H, -CONH-), 7.78 (d, J = 5.0 Hz, 1H, -CONH-), 7.59 (dd, J = 7.0, 2.0 Hz, 2H, Ar-H), 7.45 (dd, J = 7.0, 2.0 Hz, 2H, Ar-H), 7.43 (d, J = 16.0 Hz, 1H, =C-H), 7.33 (s, 1H, pyridine-H), 6.83 (dd, J = 7.5, 2.0 Hz, 1H, pyridine-H), 6.63 (d, J = 16.0 Hz, 1H, =C-H), 3.44–3.40 (m, 4H, 2 × -CH2-), 2.54 (s, 3H, CH3), 2.36 (s, 3H, CH3). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.17, 161.15, 145.27, 145.16, 137.40, 137.14, 133.89, 133.85, 129.26, 129.00, 126.43, 122.94, 115.32, 115.16, 114.55, 54.96, 38.56, 20.73, 15.71. HRMS-ESI (m/z): calcd. for C22H22O3N4F3 [M + H]+: 447.1644; found 447. 1631.
(E)-N-(2-Cinnamamidoethyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (12l): The title compound was obtained from 5l and 10 as a white solid (59%); m.p.: 197–200 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.93 (d, J = 7.0 Hz, 1H, pyridine-H), 8.30 (d, J = 5.0 Hz, 1H, -CONH-), 7.56 (d, J = 5.0 Hz, 1H, -CONH-), 7.56–7.55 (m, 3H, Ar-H and pyridine-H), 7.42–7.34 (m, 3H, Ar-H), 6.85 (dd, J = 7.0, 2.0 Hz, 1H, pyridine-H), 6.63 (d, J = 14.5 Hz, 1H, =C-H), 3.45–3.41 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.37 (s, 3H, CH3). 13C-NMR (126 MHz, CDCl3) δ (ppm): 165.36, 161.13, 145.26, 145.14, 138.73, 137.13, 134.87, 129.49, 128.97, 127.54, 126.43,122.12, 115.33, 115.16, 114.54, 44.47, 38.53, 20.73, 15.70. MS-ESI (m/z): 363 [M + H]+.
(E)-2,6-Dimethyl-N-(3-(3-p-tolylacrylamido)propyl)imidazo[1,2-a]pyridine-3-carboxamide (13a): The title compound was obtained from 6a and 9 as a white solid (52%); m.p.: 196–198 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.15 (s, 1H, pyridine-H), 7.58 (d, J = 15.5 Hz, 1H, =C-H), 7.43 (d, J = 9.0 Hz, 2H, Ar-H), 7.34 (d, J = 8.0 Hz, 1H, pyridine-H), 7.13–7.11 (m, 3H, Ar-H and pyridine-H), 6.99 (t, J = 6.0 Hz, 1H, -CONH-), 6.81 (t, J = 6.0 Hz, 1H, -CONH-), 6.43 (d, J = 15.5 Hz, 1H, =C-H),3.55 (dd, J = 12.0, 6.0 Hz, 2H, -CH2-), 3.49 (dd, J = 12.0, 6.0 Hz, 2H, -CH2-), 2.79 (s, 3H, CH3), 2.32 (s, 6H, 2 × CH3), 1.81–1.78 (m, 2H, -CH2-). 13C-NMR (126 MHz, CDCl3) δ (ppm): 165.30, 160.92, 144.67, 143.79, 139.17, 138.58, 132.15, 129.54, 127.49, 124.70, 121.96, 121.12, 115.74, 115.49, 36.48, 29.56, 20.97, 17.82, 15.59. MS-ESI (m/z): 391 [M + H]+.
(E)-2,6-Dimethyl-N-(3-(3-(3,4,5-trimethoxyphenyl)acrylamido)propyl)imidazo[1,2-a]pyridine-3-carboxamide (13b): The title compound was obtained from 6b and 9 as a white solid (48%); m.p.: 196–199 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.12 (s, 1H, pyridine-H), 7.50 (d, J =15.5 Hz, 1H, =C-H), 7.40 (d, J =9.0 Hz, 1H, pyridine-H), 7.11 (dd, J = 9.0, 1.5 Hz, 1H, Ar-H), 6.97 (t, J = 6.0 Hz, 1H, -CONH-), 6.87 (t, J = 6.0 Hz, 1H, -CONH-), 6.66 (s, 2H, Ar-H), 6.39 (d, J = 15.5 Hz, 1H, =C-H), 3.82 (s, 3H, OCH3), 3.80 (s, 6H, 2 × OCH3), 3.54–3.45 (m, 4H, 2 × -CH2-), 2.76 (s, 3H, CH3), 2.29 (s, 3H, CH3), 1.79–1.74 (m, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.27, 160.94, 153.09, 144.70, 143.82, 138.81, 138.57, 130.56, 129.15, 124.71, 121.98, 121.52, 115.75, 115.50, 104.88, 60.10, 55.86, 36.48, 29.52, 17.82, 15.69. MS-ESI (m/z): 467 [M + H]+.
(E)-N-(3-(3-(3,4-Dichlorophenyl)acrylamido)propyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (13c): The title compound was prepared from 6c and 9 as a white solid (57%); m.p.: 213–215 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.17 (s, 1H, pyridine-H), 7.55–7.54 (m, 1H, Ar-H), 7.51–7.45 (m, 2H, Ar-H and pyridine-H), 7.41 (d, J = 8.5 Hz, 1H, Ar-H), 7.30–7.27 (m, 1H, Ar-H), 7.17 (d, J = 9.0 Hz, 1H, Ar-H), 6.83 (d, J = 5.0 Hz, 1H, -CONH-), 6.76 (d, J = 5.0 Hz, 1H, -CONH-), 6.44 (d, J = 15.5 Hz, 1H, =C-H), 3.58–3.48 (m, 4H, 2 × -CH2-), 2.78 (s, 3H, CH3), 2.35 (s, 3H, CH3), 1.85–1.80 (m, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 164.68, 160.95, 144.69, 143.80, 136.07, 135.88, 131.68, 131.06, 129.44, 129.14, 127.28, 124.70, 124.47, 121.96, 115.74, 115.50, 36.59, 36.49, 29.46, 17.82, 15.69. MS-ESI (m/z): 445 [M + H]+.
(E)-N-(3-(3-(4-Methoxyphenyl)acrylamido)propyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (13d): The title compound was prepared from 6d and 9 as a white solid (45%); m.p.: 178–181 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.10 (s, 1H, pyridine-H), 7.53 (d, J = 15.5 Hz, 1H, =C-H), 7.39 (d, J = 9.0 Hz, 1H, Ar-H), 7.35 (d, J = 9.0 Hz, 1H, Ar-H), 7.10–7.05 (m, 2H, Ar-H and pyridine-H), 6.98 (t, J = 6.0 Hz, 1H, -CONH-), 6.79 (d, J = 8.5 Hz, 1H, -CONH-), 6.33 (d, J = 15.5 Hz, 1H, =C-H), 3.75 (s, 3H, OCH3), 3.53–3.44 (m, 4H, 2 × -CH2-), 2.76 (s, 3H, CH3), 2.83 (s, 3H, CH3), 1.79-1.74 (m, 2H, -CH2-). 13C-NMR (126 MHz, CDCl3) δ (ppm): 165.52, 160.94, 160.31, 144.71, 143.82, 138.38, 129.15, 129.11, 127.48, 124.73, 121.98, 119.67, 115.76, 115.50, 114.39, 55.26, 36.49, 36.46, 29.61, 17.82, 15.70. MS-ESI (m/z): 407 [M + H]+.
(E)-2,6-Dimethyl-N-(3-(3-(4-(trifluoromethyl)phenyl)acrylamido)propyl)imidazo[1,2-a]pyridine-3-carboxamide (13e): The title compound was prepared from 6e and 9 as a white solid (32%); m.p.: 162–165 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.19 (s, 1H, pyridine-H), 7.66–7.57 (m, 5H, pyridine-H and Ar-H), 7.46 (d, J = 9.5 Hz, 1H), 7.17 (dd, J = 9.0, 1.5 Hz, 1H, Ar-H), 6.76 (t, J = 6.0 Hz, 1H, -CONH-), 6.67 (d, J = 6.0 Hz, 1H, -CONH-), 6.54 (d, J = 15.5 Hz, 1H, =C-H), 3.60–3.50 (m, 4H, 2 × -CH2-), 2.80 (s, 3H, CH3), 2.35 (s, 3H, CH3), 1.86–1.81 (m, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 164.67, 161.50, 144.69, 143.80, 139.03, 136.98, 129.16, 128.17, 125.84, 125.81, 125.23, 124.97, 124.70, 121.98, 115.75, 115.51, 115.49, 36.60, 36.51, 29.46, 17.81, 15.68. MS-ESI (m/z): 445 [M + H]+.
(E)-N-(3-(3-(2-Fluorophenyl)acrylamido)propyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (13f): The title compound was prepared from 6f and 9 as a white solid (41%); m.p.: 197–199 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.99 (s, 1H, pyridine-H), 8.42 (t, J = 5.0 Hz, 1H, -CONH-), 7.86 (t, J = 5.0 Hz, 1H, -CONH-), 7.67–7.64 (m, 1H, Ar-H), 7.53–7.41 (m, 3H, Ar-H and pyridine-H), 7.30–7.22 (m, 3H, =C-H and Ar-H), 6.74 (d, J = 16.0 Hz, 1H, =C-H), 3.45–3.42 (m, 4H, 2 × -CH2-), 2.55 (s, 3H, CH3), 2.29 (s, 3H, CH3), 1.85–1.82 (m, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.13, 161.48, 161.15, 159.49, 144.90, 143.82, 131.37, 131.30, 131.16, 129.22, 129.20, 129.18, 125.05, 124.99, 124.77, 121.94, 116.21, 116.04, 115.71, 115.49, 38.87, 38.60, 29.44, 17.77, 15.62. MS-ESI (m/z): 395 [M + H]+.
(E)-N-(3-(3-(4-Fluorophenyl)acrylamido)propyl)-2,6-dimethylimidazo[1,2-a]pyridine-3-carboxamide (13g): The title compound was prepared from 6g and 9 as a white solid (67%); m.p.: 151–153 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.84 (s, 1H, pyridine-H), 8.22 (t, J = 5.5 Hz, 1H, -CONH-), 7.84 (t, J = 5.5 Hz, 1H, -CONH-), 7.64–7.61 (m, 2H, pyridine-H and Ar-H), 7.47 (d, J = 9.5 Hz, 1H, Ar-H), 7.44 (d, J = 15.5 Hz, 1H, =C-H), 7.27–7.21 (m, 3H, pyridine-H and Ar-H), 6.59 (d, J = 15.5 Hz, 1H), 3.39–3.27 (m, 4H, 2 × -CH2-), 2.58 (s, 3H, CH3), 2.30 (s, 3H, CH3), 1.79–1.73 (m, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.54, 164.10, 162.14, 161.38, 145.14, 144.25, 137.90, 132.01, 130.15, 130.08, 129.58, 125.15, 122.51, 116.44, 116.27, 116.19, 115.94, 36.96, 36.94, 29.97, 18.25, 16.13. MS-ESI (m/z): 395 [M + H]+.
(E)-2,7-Dimethyl-N-(3-(3-p-tolylacrylamido)propyl)imidazo[1,2-a]pyridine-3-carboxamide (14a): The title compound was obtained from 6a and 10 as a white solid (59%); m.p.: 197–200 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.20 (d, J = 7.0 Hz, 1H, pyridine-H), 7.56 (d, J = 15.5 Hz, 1H, =C-H), 7.32 (d, J = 7.5 Hz, 1H, Ar-H), 7.10 (d, J = 8.0 Hz, 2H, Ar-H), 6.98 (s, 2H, Ar-H), 6.68 (s, 1H, -CONH-), 6.42 (d, J = 15.5 Hz, 1H, =C-H), 3.54-3.46 (m, 4H, 2 × -CH2-), 2.77 (s, 3H, CH3), 2.38 (s, 3H, CH3), 2.31 (s, 3H, CH3), 1.77 (s, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.34, 160.96, 145.26, 144.93, 139.18, 138.61, 137.07, 132.16, 129.54, 127.50, 126.38, 121.12, 115.44, 115.16, 114.56, 36.48, 29.59, 20.96, 20.73, 15.75. MS-ESI (m/z): 391 [M + H]+.
(E)-2,7-Dimethyl-N-(3-(3-(3,4,5-trimethoxyphenyl)acrylamido)propyl)imidazo[1,2-a]pyridine-3-carboxamide (14b): The title compound was obtained from 6b and 10 as a white solid (41%); m.p.: 192–195 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.19 (d, J = 7.0 Hz, 1H, pyridine-H), 7.50 (d, J = 15.5 Hz, 1H, =C-H), 7.26 (d, J = 3.5 Hz, 1H), 6.93–6.91 (m, 2H, Ar-H), 6.69–6.67 (m, 3H, Ar-H), 6.39 (d, J = 15.5 Hz, 1H, =C-H), 3.82 (s, 3H, OCH3), 3.80 (s, 6H, 2 × OCH3), 3.54–3.45 (m, 4H, 2 × -CH2-), 2.76 (s, 3H, CH3), 2.35 (s, 3H, CH3), 1.77–1.73 (m, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.27, 160.95, 153.08, 145.25, 144.93, 138.80, 138.57, 137.08, 130.56, 126.37, 121.52, 115.42, 115.17, 114.56, 104, 88, 60.10, 55.86, 36.47, 36.44, 29.54, 20.73, 15.75. MS-ESI (m/z): 467 [M + H]+.
(E)-N-(3-(3-(3,4-Dichlorophenyl)acrylamido)propyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (14c): The title compound was prepared from 6c and 10 as a white solid (54%); m.p.: 178–181 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.27 (d, J = 7.0 Hz, 1H, pyridine-H), 7.57 (d, J = 1.5 Hz, 1H, pyridine-H), 7.52 (d, J = 15.5 Hz, 1H, =C-H), 7.44 (d, J = 8.5 Hz, 1H, Ar-H), 7.33–7.28 (m, 2H, Ar-H and -CONH-), 6.78–6.76 (m, 2H, Ar-H and -CONH-), 6.47 (d, J = 15.5 Hz, 1H, =C-H), 3.61–3.50 (m, 4H, 2 × -CH2-), 2.80 (s, 3H, CH3), 2.43 (s, 3H, CH3), 1.87–1.82 (m, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.13, 161.40, 145.68, 145.36, 137.51, 136.51, 136.32, 132.11, 132.00, 131.49, 129.88, 127.73, 126.80, 124.89, 115.86, 115.60, 114.99, 37.03, 36.92, 29.92, 21.17, 16.18. MS-ESI (m/z): 445 [M + H]+.
(E)-N-(3-(3-(4-Methoxyphenyl)acrylamido)propyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (14d): The title compound was prepared from 6d and 10 as a white solid (67%); m.p.: 217–220 °C. 1H-NMR (500 MHz, CDCl3) δ (ppm): 9.18 (d, J = 7.0 Hz, 1H, pyridine-H),7.52 (d, J = 15.5 Hz, 1H, =C-H), 7.37–7.33 (m, 2H, Ar-H and pyridine-H), 7.25 (d, J = 7.5 Hz, pyridine-H), 7.03–6.98 (m, 2H, Ar-H and -CONH-), 6.81–6.78 (m, 2H, Ar-H and -CONH-), 6.67 (dd, J = 1.5, 7.5 Hz, 1H, Ar-H), 6.48 (d, J = 16.0 Hz, 1H, =C-H), 3.76 (s, 3H), 3.52–3.44 (m, 4H, 2 × -CH2-), 2.76 (s, 3H, CH3), 2.34 (s, 3H, CH3), 1.77–1.75 (m, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.50, 160.94, 160.31, 145.25, 144.92, 138.36, 137.07, 129.10, 127.47, 119.66, 115.43, 115.17, 114.56, 114.38, 114.40, 55.20, 36.46, 36.45, 29.62, 20.73, 15.74. MS-ESI (m/z): 407 [M + H]+.
(E)-2,7-Dimethyl-N-(3-(3-(4-(trifluoromethyl)phenyl)acrylamido)propyl)imidazo[1,2-a]pyridine-3-carboxamide (14e): The title compound was prepared from 6e and 10 as a white solid (22%); m.p.: 222–225 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 9.26 (d, J = 7.0 Hz, 1H, pyridine-H), 8.38 (s, 1H, -CONH-), 7.65–7.57 (m, 4H, c and -CONH-), 7.33 (s, 1H, pyridine-H), 6.77–6.65 (m, 3H, Ar-H), 6.52 (d, J = 15.5 Hz, 1H, =C-H), 3.59–3.51 (m, 4H, 2 × -CH2-), 2.80 (s, 3H, CH3), 2.42 (s, 3H, CH3), 1.83–1.80 (m, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 164.67, 161.50, 144.69, 143.80, 139.03, 136.98, 129.16, 128.17, 125.84, 125.81, 125.23, 124.97, 124.70, 121.98, 115.75, 115.51, 115.49, 36.60, 36.51, 29.46, 17.81, 15.68. MS-ESI (m/z): 445 [M + H]+.
(E)-N-(3-(3-(2-Fluorophenyl)acrylamido)propyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (14f): The title compound was prepared from 6f and 9 as a white solid (34%); m.p.: 197–199 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.93 (d, J = 7.0 Hz, 1H, pyridine-H), 8.45 (d, J = 5.0 Hz, 1H, -CONH-), 7.78 (d, J = 5.0 Hz, 1H, -CONH-), 7.67–7.62 (m, 1H, Ar-H), 7.51 (d, J = 16.0 Hz, 1H, =C-H), 7.43–7.41 (m, 1H, Ar-H), 7.33 (s, 1H, pyridine-H), 7.29–7.23 (m, 2H, Ar-H), 6.81 (dd, J = 7.5, 1.5 Hz, 1H, Ar-H), 6.74 (d, J = 16.0 Hz, 1H, =C-H), 3.45–3.42 (m, 4H, 2 × -CH2-), 2.54 (s, 3H, CH3), 2.28 (s, 3H, CH3), 1.85–1.82 (m, 2H, -CH2-). 13C-NMR (126 MHz, DMSO-d6) δ (ppm): 165.19, 161.92, 161.60, 159.93, 145.72, 145.60, 137.55, 131.78, 131.61, 129.65, 129.63, 126.87, 125.47, 125.44, 125.42, 116.64, 116.47, 115.78, 115.57, 114.98, 38.88, 38.61, 29.44, 17.76, 15.62. MS-ESI (m/z): 395 [M + H]+.
(E)-N-(3-(3-(4-Fluorophenyl)acrylamido)propyl)-2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide (14g): The title compound was prepared from 6g and 10 as a white solid (55%); m.p.: 203–206 °C. 1H-NMR (500 MHz, DMSO-d6) δ (ppm): 8.92 (d, J = 7.5 Hz, 1H, pyridine-H), 8.22 (d, J = 5.5 Hz, 1H, -CONH-), 7.77 (d, J = 5.5 Hz, 1H, -CONH-), 7.64–7.60 (m, 1H, Ar-H), 7.42 (d, J = 16.0 Hz, 1H, =C-H), 7.41 (s, 1H, pyridine-H), 7.26–7.22 (m, 2H, Ar-H), 6.84 (dd, J = 7.0, 1.5 Hz, 1H, Ar-H), 6.58 (d, J = 16.0 Hz, 1H, =C-H), 3.37–3.26 (m, 4H, 2 × -CH2-), 2.57 (s, 3H, CH3), 2.35 (s, 3H, CH3), 1.78–1.72 (m, 2H, -CH2-). 13C-NMR (126 MHz, CDCl3) δ (ppm): 165.10, 163.66, 161.70, 160.95, 145.25, 144.92, 137.45, 137.07, 131.57, 131.55, 129.72, 129.65, 122.08, 116.00, 115.83, 115.43, 115.16, 114.56, 36.51, 36.48, 29.55, 20.73, 15.74. MS-ESI (m/z): 395 [M + H]+.