Silymarin Constituents Enhance ABCA1 Expression in THP-1 Macrophages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impact of the Studied Components of Silymarin on THP-1 Cell Viability
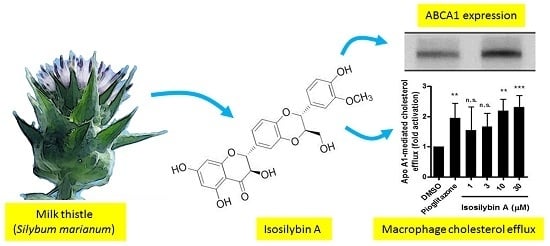
2.2. Impact of Isosilybin A on Cholesterol Efflux



2.3. Impact of the Active Components of Silymarin on ABCA1 Protein Expression

3. Materials and Methods
3.1. Chemicals, Cell Culture Reagents and Antibodies
3.2. Sources of the Compounds
3.3. Cell Culture
3.4. Resazurin Conversion Assay
3.5. ABCA1 Protein Quantification
3.6. Cholesterol Efflux Assay
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Kvasnicka, F.; Biba, B.; Sevcik, R.; Voldrich, M.; Kratka, J. Analysis of the active components of silymarin. J. Chromatogr. A 2003, 990, 239–245. [Google Scholar] [CrossRef]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and silymarin—New and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, A.E. Dihydroquercetin: More than just an impurity? Eur. J. Pharmacol. 2012, 684, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.W.; Liu, Y.Z. Molecular structure and stereochemistry of silybin a, silybin b, isosilybin a, and isosilybin b, isolated from silybum marianum (milk thistle). J. Nat. Prod. 2003, 66, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Saller, R.; Meier, R.; Brignoli, R. The use of silymarin in the treatment of liver diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef] [PubMed]
- Skottova, N.; Krecman, V. Silymarin as a potential hypocholesterolaemic drug. Physiol. Res. 1998, 47, 1–7. [Google Scholar] [PubMed]
- Krecman, V.; Skottova, N.; Walterova, D.; Ulrichova, J.; Simanek, V. Silymarin inhibits the development of diet-induced hypercholesterolemia in rats. Planta Med. 1998, 64, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Skottova, N.; Vecera, R.; Urbanek, K.; Vana, P.; Walterova, D.; Cvak, L. Effects of polyphenolic fraction of silymarin on lipoprotein profile in rats fed cholesterol-rich diets. Pharmacol. Res. 2003, 47, 17–26. [Google Scholar] [CrossRef]
- Sobolova, L.; Skottova, N.; Vecera, R.; Urbanek, K. Effect of silymarin and its polyphenolic fraction on cholesterol absorption in rats. Pharmacol. Res. 2006, 53, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.W.; Navarro, V.J.; Afdhal, N.; Belle, S.H.; Wahed, A.S.; Hawke, R.L.; Doo, E.; Meyers, C.M.; Reddy, K.R. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis c unsuccessfully treated with interferon therapy: A randomized controlled trial. JAMA 2012, 308, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Lawn, R.M. Abca1: The gatekeeper for eliminating excess tissue cholesterol. J. Lipid Res. 2001, 42, 1173–1179. [Google Scholar] [PubMed]
- Oram, J.F. Tangier disease and abca1. BBA Mol. Cell Biol. Lipids 2000, 1529, 321–330. [Google Scholar] [CrossRef]
- Van Dam, M.J.; de Groot, E.; Clee, S.M.; Hovingh, G.K.; Roelants, R.; Brooks-Wilson, A.; Zwinderman, A.H.; Smit, A.J.; Smelt, A.H.M.; Groen, A.K.; et al. Association between increased arterial-wall thickness and impairment in abca1-driven cholesterol efflux: An observational study. Lancet 2002, 359, 37–41. [Google Scholar] [CrossRef]
- Phillips, M.C. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 2014, 289, 24020–24029. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Boisvert, W.A.; Lee, C.H.; Laffitte, B.A.; Barak, Y.; Joseph, S.B.; Liao, D.; Nagy, L.; Edwards, P.A.; Curtiss, L.K.; et al. A ppar gamma-lxr-abca1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 2001, 7, 161–171. [Google Scholar] [CrossRef]
- Pferschy-Wenzig, E.M.; Atanasov, A.G.; Malainer, C.; Noha, S.M.; Kunert, O.; Schuster, D.; Heiss, E.H.; Oberlies, N.H.; Wagner, H.; Bauer, R.; et al. Identification of isosilybin a from milk thistle seeds as an agonist of peroxisome proliferator-activated receptor gamma. J. Nat. Prod. 2014, 77, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (ppar-gamma): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, A.; Atanasov, A.G.; Heiss, E.H.; Baumgartner, L.; Schwaiger, S.; Rollinger, J.M.; Stuppner, H.; Dirsch, V.M. 2-(2,4-dihydroxyphenyl)-5-(E)-propenylbenzofuran promotes endothelial nitric oxide synthase activity in human endothelial cells. Biochem. Pharmacol. 2012, 84, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, H.; Ayaori, M.; Iizuka, M.; Terao, Y.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Uehara, Y.; et al. Pioglitazone enhances cholesterol efflux from macrophages by increasing abca1/abcg1 expressions via ppar gamma/lxr alpha pathway: Findings from in vitro and ex vivo studies. Atherosclerosis 2011, 219, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Rotter, S.; Ladurner, A.; Heiss, E.H.; Oberlies, N.H.; Dirsch, V.M.; Atanasov, A.G. Silymarin Constituents Enhance ABCA1 Expression in THP-1 Macrophages. Molecules 2016, 21, 55. https://doi.org/10.3390/molecules21010055
Wang L, Rotter S, Ladurner A, Heiss EH, Oberlies NH, Dirsch VM, Atanasov AG. Silymarin Constituents Enhance ABCA1 Expression in THP-1 Macrophages. Molecules. 2016; 21(1):55. https://doi.org/10.3390/molecules21010055
Chicago/Turabian StyleWang, Limei, Susanne Rotter, Angela Ladurner, Elke H. Heiss, Nicholas H. Oberlies, Verena M. Dirsch, and Atanas G. Atanasov. 2016. "Silymarin Constituents Enhance ABCA1 Expression in THP-1 Macrophages" Molecules 21, no. 1: 55. https://doi.org/10.3390/molecules21010055
APA StyleWang, L., Rotter, S., Ladurner, A., Heiss, E. H., Oberlies, N. H., Dirsch, V. M., & Atanasov, A. G. (2016). Silymarin Constituents Enhance ABCA1 Expression in THP-1 Macrophages. Molecules, 21(1), 55. https://doi.org/10.3390/molecules21010055