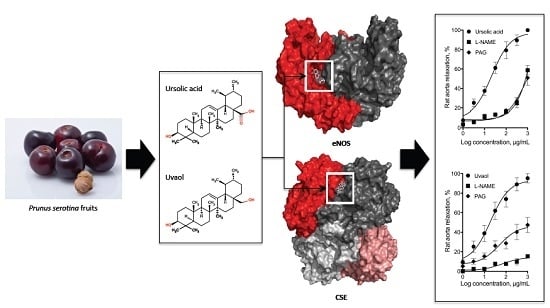
Role of Nitric Oxide and Hydrogen Sulfide in the Vasodilator Effect of Ursolic Acid and Uvaol from Black Cherry Prunus serotina Fruits
Abstract
:1. Introduction
2. Results
2.1. Bio-Directed Chemical Study of the Dichloromethane Extract Obtained from the Fruits of P. serotina


| 1H-NMR (400 MHz, Acetone) δ (ppm) | δH 5.22 (1H, m, H-12), 3.15 (1H, m, H-3), 2.25 (1H, d, H-18), 1.13 (3H, s, H-23), 0.98 (3H, s, H-27), 0.96 (3H, s, H-26), 0.95 (3H, s, H-24), 0.89 (3H, d, H-29), 0.84 (3H, d, H-30), 0.78 (3H, s, H-25) |
| 13C-NMR (400 MHz, Acetone) δ (ppm) | δC 177.6 (C-28), 138.4 (C-13), 125.3 (C-12), 77.7 (C-3), 55.3 (C-5), 52.9 (C-18), 47.5 (C-9), 47.3 (C-17), 41.9 (C-14), 39.5 (C-8), 38.9 (C-19), 38.9 (C-4), 38.6 (C-1), 36.8 (C-22), 36.7 (C-10), 33.0 (C-7), 30.4 (C-21), 27.9 (C-15), 27.8 (C-2), 24.1 (C-16), 23.1 (C-27), 23.0 (C-11), 20.5 (C-30), 18.2 (C-6), 16.8 (C-26), 16.6 (C-24), 15.4 (C-29), 15.0 (C-25) |
| 1H-NMR (400 MHz, DMSO-d6) δ (ppm) | δH 5.13 (1H, t, H-12), 3.80 (1H, d, H-20a), 3.38 (1H, d, H-28b), 3.20 (1H, d, H-3), 1.05 (3H, s, CH3), 0.98 (3H, s, CH3), 0.97 (3H, s, CH3), 0.96 (3H, s, CH3), 0.93 (3H, d, CH3), 0.83 (3H, d, CH3), 0.80 (3H, s, CH3). |
| 13C-NMR (100 MHz, DMSO-d6) δ (ppm) | δC 177.8 (C-28),138.7 (C-13), 125.1 (C-12), 77.1 (C-3), 58.7 (C-18), 52.3 (C-5), 41.5 (C-14).17.9 (C-29), 15.9 (C-23). |
2.2. Elucidation of the Mechanism of Action of Ursolic Acid and Uvaol








3. Discussion
4. Materials and Methods
4.1. Plant Material and Reagents
4.2. Experimental Animals
4.3. Collection and Preservation of Plant Material
4.4. Bio-Directed Chemical Study of the Dichloromethane Extract Obtained from the Fruits of P. serotina
4.4.1. Preparation of the Dichloromethane Extract from the Fruits of P. serotina
4.4.2. Bio-Directed Fractionation of the Dichloromethane Extract and Purification of Ursolic Acid and Uvaol
4.4.3. Determination of the Chemical Structure of Ursolic Acid and Uvaol
4.5. Determination of the Vasodilator Effect and Elucidation of the Mechanism of Action of Ursolic Acid and Uvaol
4.5.1. Isolated Rat Aorta Assay
4.5.2. Participation of the Endothelium in the Vasodilator Response
4.5.3. Evaluation of the Participation of the NO/cGMP and the H2S/KATP Channel Pathways in the Vasodilator Response
4.6. Increase in NO and H2S Levels Elicited by Ursolic Acid and Uvaol
4.6.1. NOS Enzymatic Activity Assay
4.6.2. CSE Enzymatic Activity Assay
4.7. In silico Studies
4.7.1. Protein Preparation
4.7.2. Ligand Preparation
4.7.3. Docking Studies
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. The Top 10 Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 10 January 2014).
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Velázquez Monroy, Ó.; Barinagarrementería Aldatz, F.S.; Rubio Guerra, A.F.; Verdejo, J.; Méndez Bello, M.Á.; Violante, R.; Pavía, A.; Alvarado-Ruiz, R.; Lara Esqueda, A. Morbilidad y mortalidad de la enfermedad isquémica del corazón y cerebrovascular en México. 2005. Arch. Cardiol. Méx. 2007, 77, 31–39. [Google Scholar]
- INEGI. Causas de Defunción en México 2013. Available online: http://www3.inegi.org.mx/sistemas/sisept/Default.aspx?t=mdemo107&s=est&c=23587 (accessed on 12 February 2014).
- Borja-Aburto, V.H.; Gonzalez-Anaya, J.A.; Davila-Torres, J.; Rascon-Pacheco, R.A.; Gonzalez-Leon, M. Evaluation of the impact on non-communicable chronic diseases of a major integrated primary health care program in Mexico. Fam. Pract. 2015. [Google Scholar] [CrossRef]
- Wu, F.; Guo, Y.; Chatterji, S.; Zheng, Y.; Naidoo, N.; Jiang, Y.; Biritwum, R.; Yawson, A.; Minicuci, N.; Salinas-Rodriguez, A.; et al. Common risk factors for chronic non-communicable diseases among older adults in China, Ghana, Mexico, India, Russia and South Africa: The study on global ageing and adult health (sage) wave 1. BMC Public Health 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.; Sarafidis, P.; Agarwal, R.; Ruilope, L. Review of blood pressure control rates and outcomes. J. Am. Soc. Hypertens. 2014, 8, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Samuel, S.M.; Ravishankar, S.; Marei, I.; Arunachalam, G.; Ding, H. The endothelium: Influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharmacol. 2012, 90, 713–738. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.T. Endothelium-derived relaxing factors of small resistance arteries in hypertension. Toxicol. Res. 2014, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Szabo, C.; Ichinose, F.; Ahmed, A.; Whiteman, M.; Papapetropoulos, A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol. Sci. 2015, 36, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Schlaich, M.; Esler, M. New drugs, procedures, and devices for hypertension. Lancet 2012, 380, 591–600. [Google Scholar] [CrossRef]
- Galie, N.; Ghofrani, A.H. New horizons in pulmonary arterial hypertension therapies. Eur. Respir. Rev. 2013, 22, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Hydrogen sulfide: The third gasotransmitter in biology and medicine. Antioxid. Redox Signal. 2010, 12, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S. Application of nitric oxide in drug discovery and development. Expert Opin. Drug Discov. 2011, 6, 1139–1154. [Google Scholar] [CrossRef] [PubMed]
- Kots, A.Y.; Bian, K.; Murad, F. Nitric oxide and cyclic GMP signaling pathway as a focus for drug development. Curr. Med. Chem. 2011, 18, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Evora, P.R.; Evora, P.M.; Celotto, A.C.; Rodrigues, A.J.; Joviliano, E.E. Cardiovascular therapeutics targets on the NO-sGC-cGMP signaling pathway: A critical overview. Curr. Drug Targets 2012, 13, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, K.M.; Faas, M.M.; van Goor, H.; Lely, A.T. Gasotransmitters a solution for the therapeutic dilemma in preeclampsia? Hypertension 2013, 62, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Russwurm, M.; Russwurm, C.; Koesling, D.; Mergia, E. NO/cGMP: The past, the present, and the future. In Guanylate Cyclase and Cyclic GMP: Methods and Protocols; Springer Science + Business Media, LLC: New York, NY, USA, 2013; pp. 1–16. [Google Scholar]
- Bełtowski, J. Hydrogen sulfide in pharmacology and medicine—An update. Pharmacol. Rep. 2015, 67, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Bowman, L.; D’Arsigny, C.L.; Archer, S.L. Soluble guanylate cyclase: A new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clin. Pharmacol. Ther. 2015, 97, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, K.M.; Karumanchi, S.A.; Lely, A.T. Hydrogen sulfide: Role in vascular physiology and pathology. Curr. Opin. Nephrol. Hypertens. 2015, 24, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pacheco, A.; Xian, M. Medicinal chemistry: Insights into the development of novel H2S donors. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Springer International Publishing: Cham, Switzerland, 2015; pp. 365–388. [Google Scholar]
- Ibarra-Alvarado, C.; Rojas, A.; Mendoza, S.; Bah, M.; Gutiérrez, D.; Hernández-Sandoval, L.; Martínez, M. Vasoactive and antioxidant activities of plants used in Mexican traditional medicine for the treatment of cardiovascular diseases. Pharm. Biol. 2010, 48, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Alvarado, C.; Rojas, A.; Luna, F.; Rojas, J.I.; Rivero-Cruz, B.; Rivero-Cruz, J.F. Vasorelaxant constituents of the leaves of Prunus serotina “capulín”. Rev. Latinoam. Quím. 2009, 37, 164–173. [Google Scholar]
- De la Villa Torre, F.L.-V.F.; Ibarra-Alvarado, C.; Rivero-Cruz, F.; Rojas-Molina, J.I.; Rojas-Molina, A. Innovations in Food Science and Food Biotechnology in Developing Countries; Regalado, C.G., García, B.E., Eds.; Asociación Mexicana de Ciencias de los Alimentos: Mexico, D.F., Mexico, 2010; pp. 13–25. [Google Scholar]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, J.I.; Yahia, E.M.; Rivera-Pastrana, D.M.; Rojas-Molina, A.; Zavala-Sánchez, Á.M. Nutraceutical value of black cherry Prunus serotina ehrh. Fruits: Antioxidant and antihypertensive properties. Molecules 2013, 18, 14597–14612. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants-rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Guinda, A.N.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic triterpenoids from olive fruit and leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef] [PubMed]
- Wolbis, M.; Olszewska, M.; Wesolowski, W.J. Triterpenes and sterols in the flowers and leaves of Prunus spinosa L.(rosaceae). Acta Poloniae Pharm. 2001, 58, 459–462. [Google Scholar] [PubMed]
- Amico, V.; Barresi, V.; Condorelli, D.; Spatafora, C.; Tringali, C. Antiproliferative terpenoids from almond hulls (Prunus dulcis): Identification and structure-activity relationships. J. Agric. Food Chem. 2006, 54, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Marcia, M.; Konorov, S.O.; Schulze, H.G.; Blades, M.W.; Turner, R.F.; Jetter, R. In situ analysis by microspectroscopy reveals triterpenoid compositional patterns within leaf cuticles of Prunus laurocerasus. Planta 2008, 227, 823–834. [Google Scholar]
- Kadu, C.A.; Parich, A.; Schueler, S.; Konrad, H.; Muluvi, G.M.; Eyog-Matig, O.; Muchugi, A.; Williams, V.L.; Ramamonjisoa, L.; Kapinga, C. Bioactive constituents in Prunus africana: Geographical variation throughout Africa and associations with environmental and genetic parameters. Phytochemistry 2012, 83, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-T.; Lee, S.-H.; Li, W.; Jang, H.-D.; Kim, Y.-H. Terpenes and sterols from the fruits of Prunus mume and their inhibitory effects on osteoclast differentiation by suppressing tartrate-resistant acid phosphatase activity. Arch. Pharm. Res. 2015, 38, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J. Enzyme Inhib. Med. Chem. 2011, 26, 616–642. [Google Scholar] [CrossRef] [PubMed]
- Camer, D.; Yu, Y.; Szabo, A.; Huang, X.F. The molecular mechanisms underpinning the therapeutic properties of oleanolic acid, its isomer and derivatives for type 2 diabetes and associated complications. Mol. Nutr. Food Res. 2014, 58, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Yoon, S.Y.; Roh, D.H.; Jeon, M.J.; Seo, H.S.; Uh, D.K.; Kwon, Y.B.; Kim, H.W.; Han, H.J.; Lee, H.J. The anti-arthritic effect of ursolic acid on zymosan-induced acute inflammation and adjuvant-induced chronic arthritis models. J. Pharm. Pharmacol. 2008, 60, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Forstermann, U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr. Pharm. Des. 2009, 15, 3133–3145. [Google Scholar] [CrossRef] [PubMed]
- Allouche, Y.; Beltrán, G.; Gaforio, J.J.; Uceda, M.; Mesa, M.D. Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem. Toxicol. 2010, 48, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, C.; López-Biedma, A.; Gaforio, J.J. The differential localization of a methyl group confers a different anti-breast cancer activity to two triterpenes present in olives. Food Funct. 2015, 6, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Crespo, F.; Vergara-Galicia, J.; Villalobos-Molina, R.; López-Guerrero, J.J.; Navarrete-Vázquez, G.; Estrada-Soto, S. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci. 2006, 79, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.Y.; López-Martínez, S.; López-Vallejo, F.; Medina-Franco, J.L.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Navarrete-Vazquez, G.; Hidalgo-Figueroa, S.; Hernández-Abreu, O.; Estrada-Soto, S. Vasorelaxant activity of some structurally related triterpenic acids from Phoradendron reichenbachianum (Viscaceae) mainly by no production: Ex vivo and in silico studies. Fitoterapia 2012, 83, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Perona, J.S.; Herrera, M.D.; Ruiz-Gutierrez, V. Triterpenic compounds from “orujo” olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats. J. Agric. Food Chem. 2006, 54, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Török, J.; Kristek, F. Functional and morphological pattern of vascular responses in two models of experimental hypertension. Exp. Clin. Cardiol. 2001, 6, 142–148. [Google Scholar] [PubMed]
- Torok, J.; Koprdová, R.; Cebová, M.; Kunes, J.; Kristek, F. Functional and structural pattern of arterial responses in hereditary hypertriglyceridemic and spontaneously hypertensive rats. Physiol. Res. 2006, 55, S65–S71. [Google Scholar] [PubMed]
- Ajay, M.; Achike, F.I.; Mustafa, M.R. Modulation of vascular reactivity in normal, hypertensive and diabetic rat aortae by a non-antioxidant flavonoid. Pharmacol. Res. 2007, 55, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J.; Jamroz-Wiśniewska, A. Hydrogen sulfide and endothelium-dependent vasorelaxation. Molecules 2014, 19, 21183–21199. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Sikka, G.; Gazi, S.K.; Steppan, J.; Jung, S.M.; Bhunia, A.K.; Barodka, V.M.; Gazi, F.K.; Barrow, R.K.; Wang, R. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, M.; Fukuda, R.; Bateman, R.M.; Yamamoto, T.; Suematsu, M. Interactions of multiple gas-transducing systems: Hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal. 2010, 13, 157–192. [Google Scholar] [CrossRef] [PubMed]
- Berenyiova, A.; Grman, M.; Mijuskovic, A.; Stasko, A.; Misak, A.; Nagy, P.; Ondriasova, E.; Cacanyiova, S.; Brezova, V.; Feelisch, M. The reaction products of sulfide and s-nitrosoglutathione are potent vasorelaxants. Nitric Oxide 2015, 46, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Fernandez, B.O.; Kelm, M.; Butler, A.R.; Feelisch, M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide 2015, 46, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.; Pou, S.; Rosen, G.M. Oxygen reduction by nitric-oxide synthases. J. Biol. Chem. 2001, 276, 14533–14536. [Google Scholar] [CrossRef] [PubMed]
- Fischmann, T.O.; Hruza, A.; da Niu, X.; Fossetta, J.D.; Lunn, C.A.; Dolphin, E.; Prongay, A.J.; Reichert, P.; Lundell, D.J.; Narula, S.K. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat. Struct. Mol. Biol. 1999, 6, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.; Li, H.; Martásek, P.; Southan, G.; Masters, B.S.S.; Poulos, T.L. Crystal structure of nitric oxide synthase bound to nitro indazole reveals a novel inactivation mechanism. Biochemistry 2001, 40, 13448–13455. [Google Scholar] [CrossRef]
- Alderton, W.; Cooper, C.; Knowles, R. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, H.; Flinspach, M.; Poulos, T.L.; Silverman, R.B. Computer modeling of selective regions in the active site of nitric oxide synthases: Implication for the design of isoform-selective inhibitors. J. Med. Chem. 2003, 46, 5700–5711. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J.; Santolini, J.; Wang, Z.-Q.; Wei, C.-C.; Adak, S. Update on mechanism and catalytic regulation in the NO synthases. J. Biol. Chem. 2004, 279, 36167–36170. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Collins, R.; Huang, S.; Holmberg-Schiavone, L.; Anand, G.S.; Tan, C.-H.; van-den-Berg, S.; Deng, L.-W.; Moore, P.K.; Karlberg, T. Structural basis for the inhibition mechanism of human cystathionine γ-lyase, an enzyme responsible for the production of H2S. J. Biol. Chem. 2009, 284, 3076–3085. [Google Scholar] [CrossRef] [PubMed]
- Mallavadhani, U.; Panda, A.K.; Rao, Y. Diospyros melanoxylon leaves: A rich source of pentacyclic triterpenes. Pharm. Biol. 2001, 39, 20–24. [Google Scholar] [CrossRef]
- Sang, S.; Lapsley, K.; Rosen, R.T.; Ho, C.-T. New prenylated benzoic acid and other constituents from almond hulls (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Feelisch, M.; Kotsonis, P.; Siebe, J.; Clement, B.; Schmidt, H.H. The soluble guanylyl cyclase inhibitor 1H-[1,2,4] oxadiazolo [4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome p-450 enzymes involved in nitric oxide donor bioactivation. Mol. Pharmacol. 1999, 56, 243–253. [Google Scholar] [PubMed]
- Ibarra-Alvarado, C.; García, J.A.; Aguilar, M.B.; Rojas, A.; Falcón, A.; de la Cotera, E.P.H. Biochemical and pharmacological characterization of toxins obtained from the fire coral Millepora complanata. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Galle, J.; Zabel, U.; Hübner, U.; Hatzelmann, A.; Wagner, B.; Wanner, C.; Schmidt, H.H. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br. J. Pharmacol. 1999, 127, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Alvarado, C.; Galle, J.; Melichar, V.O.; Mameghani, A.; Schmidt, H.H. Phosphorylation of blood vessel vasodilator-stimulated phosphoprotein at serine 239 as a functional biochemical marker of endothelial nitric oxide/cyclic GMP signaling. Mol. Pharmacol. 2002, 61, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-L.; Chang, J.-S.; Yu, W.-Y.; Cheah, K.-P.; Li, J.-S.; Cheng, H.-W.; Hu, C.-M. Polygonum viviparum L. Induces vasorelaxation in the rat thoracic aorta via activation of nitric oxide synthase in endothelial cells. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Guevara, I.; Iwanejko, J.; Dembińska-Kieć, A.; Pankiewicz, J.; Wanat, A.; Anna, P.; Goła̧bek, I.; Bartuś, S.; Malczewska-Malec, M.; Szczudlik, A. Determination of nitrite/nitrate in human biological material by the simple Griess reaction. Clin. Chim. Acta 1998, 274, 177–188. [Google Scholar] [CrossRef]
- Cheung, S.H.; Kwok, W.K.; To, K.F.; Lau, J.Y.W. Anti-atherogenic effect of hydrogen sulfide by over-expression of cystathionine gamma-lyase (CSE) gene. PLoS ONE 2014, 9, e113038. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.; Papapetropoulos, A.; Vellecco, V.; Zhou, Z.; Pyriochou, A.; Roussos, C.; Roviezzo, F.; Brancaleone, V.; Cirino, G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Bolton, E.E.; Wang, Y.; Thiessen, P.A.; Bryant, S.H. Pubchem: Integrated platform of small molecules and biological activities. Annu. Rep. Comput. Chem. 2008, 4, 217–241. [Google Scholar]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of ursolic acid and uvaol are not available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Romo-Mancillas, A.; López-Vallejo, F.H.; Solís-Gutiérrez, M.; Rojas-Molina, J.I.; Rivero-Cruz, F. Role of Nitric Oxide and Hydrogen Sulfide in the Vasodilator Effect of Ursolic Acid and Uvaol from Black Cherry Prunus serotina Fruits. Molecules 2016, 21, 78. https://doi.org/10.3390/molecules21010078
Luna-Vázquez FJ, Ibarra-Alvarado C, Rojas-Molina A, Romo-Mancillas A, López-Vallejo FH, Solís-Gutiérrez M, Rojas-Molina JI, Rivero-Cruz F. Role of Nitric Oxide and Hydrogen Sulfide in the Vasodilator Effect of Ursolic Acid and Uvaol from Black Cherry Prunus serotina Fruits. Molecules. 2016; 21(1):78. https://doi.org/10.3390/molecules21010078
Chicago/Turabian StyleLuna-Vázquez, Francisco J., César Ibarra-Alvarado, Alejandra Rojas-Molina, Antonio Romo-Mancillas, Fabián H. López-Vallejo, Mariana Solís-Gutiérrez, Juana I. Rojas-Molina, and Fausto Rivero-Cruz. 2016. "Role of Nitric Oxide and Hydrogen Sulfide in the Vasodilator Effect of Ursolic Acid and Uvaol from Black Cherry Prunus serotina Fruits" Molecules 21, no. 1: 78. https://doi.org/10.3390/molecules21010078
APA StyleLuna-Vázquez, F. J., Ibarra-Alvarado, C., Rojas-Molina, A., Romo-Mancillas, A., López-Vallejo, F. H., Solís-Gutiérrez, M., Rojas-Molina, J. I., & Rivero-Cruz, F. (2016). Role of Nitric Oxide and Hydrogen Sulfide in the Vasodilator Effect of Ursolic Acid and Uvaol from Black Cherry Prunus serotina Fruits. Molecules, 21(1), 78. https://doi.org/10.3390/molecules21010078