Porphyrin-Based Metal-Organic Frameworks as Heterogeneous Catalysts in Oxidation Reactions
Abstract
:1. Introduction
2. Por-MOFs as Heterogeneous Catalysts in Oxidation Reactions
2.1. Oxidation of Alkanes
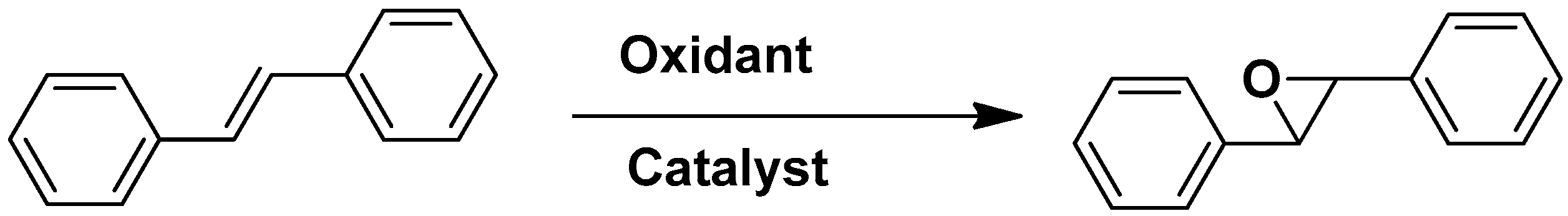
2.2. Oxidation of Alkenes
2.3. Oxidation of Phenols
3. Outlook
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | Coordination Polymer |
| CP-450 | Cytochrome P-450 |
| CZJ | Chemistry Department of Zhejiang University |
| DCDBP | 5,15-bis(2,6-dibromophenyl)-10,20-bis(3,5-dicarboxyphenyl)porphyrin |
| DPNI | N,Nʹ-di(4-pyridyl)-1,4,5,8-naphthalenetetracarboxydiimide |
| F10DPyP | 5,15-bis(pentafluorophenyl)-10,20-di(pyridyl)porphyrin |
| F5CPP | 5,10,15-tris(p-carboxyphenyl)-20-(pentafluorophenyl)porphyrin |
| F10CPP | 5,15- bis(p-carboxyphenyl)-10,20-bis(pentafluorophenyl)porphyrin |
| H8OCPP | 5,10,15,20-tetrakis(3,5-dicarboxylphenyl)porphyrin |
| MMPF | Metal-Metalloporphyrin Frameworks |
| MOFs | Metal-Organic Frameworks |
| PIZA | Porphyrinic Illinois Zeolite Analogue |
| Por-MOFs | Porphyrin-based MOFs |
| RPMs | Robust Porphyrinic Materials |
| SBUs | Secondary Building Units |
| TNPP | 5,10,15,20-tetrakis[p-(nicotinoyloxy)phenyl]porphyrin |
| TpCPP | 5,10,15,20-tetrakis(p-carboxyphenyl)porphyrin |
| TON | Turnover Number |
| ZJU | Zheijang University |
References
- Hoskins, B.F.; Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3-D-linked molecular rods. A reappraisal of the Zn(CN)2 and Cd(CN)2 structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4] [CuIZnII(CN)4] and CuI[4,4′,4′′,4′′′-tetracyanotetraphenylmethane]BF4.xC6H5NO2. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar]
- Yaghi, O.M.; Li, G.M.; Li, H.L. Selective binding and removal of guests in a microporous metal-organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Cook, T.R.; Zheng, Y.R.; Stang, P.J. Metal-organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal-organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Hoskins, B.F.; Michail, D.M.; Robson, R. Assembly of porphyrin building-blocks into network structures with large channels. Nature 1994, 369, 727–729. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. Handbook of Porphyrin Science; World Scientific Publisher: Singapore, 2010–2014; Volumes 1–35. [Google Scholar]
- Meunier, B.; de Visser, S.P.; Shaik, S. Mechanism of oxidation reactions catalyzed by cytochrome P-450 enzymes. Chem. Rev. 2004, 104, 3947–3980. [Google Scholar] [CrossRef] [PubMed]
- Shaik, S.; Cohen, S.; Wang, Y.; Chen, H.; Kumar, D.; Thiel, W. P-450 Enzymes: Their structure, reactivity, and selectivity-modeled by QM/MM calculations. Chem. Rev. 2010, 110, 949–1017. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, D. Cytochromes P-450 and model systems: Great diversity of catalyzed reactions. Pure Appl. Chem. 1994, 66, 737–744. [Google Scholar] [CrossRef]
- Groves, J.T. The bioinorganic chemistry of iron in oxygenases and supramolecular assemblies. Proc. Natl. Acad. Sci. USA 2003, 100, 3569–3574. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, D. A brief history of the contribution of metalloporphyrin models to cytochrome P-450 chemistry and oxidation catalysis. C. R. Chim. 2007, 10, 392–413. [Google Scholar] [CrossRef]
- Fukushima, M.; Mizutani, Y.; Maeno, S.; Zhu, Q.Q.; Kuramitz, H.; Nagao, S. Influence of halogen substituents on the catalytic oxidation of 2,4,6-halogenated phenols by Fe(III)-tetrakis(p-hydroxyphenyl) porphyrins and potassium monopersulfate. Molecules 2012, 17, 48–60. [Google Scholar] [CrossRef] [PubMed]
- White, R.E. Cytochrome P-450 - structure, mechanism, and biochemistry. Science 1986, 234, 884. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Tanabe, Y.; Morimoto, K.; Tatsumi, K. Role of humic acid fraction with higher aromaticity in enhancing the activity of a biomimetic catalyst, tetra(p-sulfonatophenyl)porphineiron(III). Biomacromolecules 2007, 8, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Groves, J.T. Enzymatic C-H bond activation using push to get pull. Nat. Chem. 2014, 6, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Mansuy, D. Activation of alkanes - the biomimetic approach. Coord. Chem. Rev. 1993, 125, 129–141. [Google Scholar] [CrossRef]
- Shelnutt, J.A.; Song, X.Z.; Ma, J.G.; Jia, S.L.; Jentzen, W.; Medforth, C.J. Nonplanar porphyrins and their significance in proteins. Chem. Soc. Rev. 1998, 27, 31–41. [Google Scholar] [CrossRef]
- Perry, J.J.; Perman, J.A.; Zaworotko, M.J. Design and synthesis of metal-organic frameworks using metal-organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 2009, 38, 1400–1417. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Wu, C.-D. Functional porphyrinic metal-organic frameworks: Crystal engineering and applications. Dalton Trans. 2012, 41, 3879–3888. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.-Y.; Chrzanowski, M.; Ma, S. Metal-metalloporphyrin frameworks: A resurging class of functional materials. Chem. Soc. Rev. 2014, 43, 5841–5866. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, B. Recent developments in metal-metalloporphyrin frameworks. Dalton Trans. 2015, 44, 14574–14583. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Kim, S.J.; Kim, Y. Porphyrinic metal-organic frameworks from custom-designed porphyrins. Cryst. Eng. Comm. 2016, 18, 345–368. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Hoskins, B.F.; Robson, R. A new type of infinite 3D Polymeric network containing 4-connected, peripherally linked metalloporphyrin building-blocks. J. Am. Chem. Soc. 1991, 113, 3606–3607. [Google Scholar] [CrossRef]
- Suslick, K.S.; Bhyrappa, P.; Chou, J.H.; Kosal, M.E.; Nakagaki, S.; Smithenry, D.W.; Wilson, S.R. Microporous porphyrin solids. Acc. Chem. Res. 2005, 38, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Shultz, A.M.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Active-site-accessible, porphyrinic metal-organic framework materials. J. Am. Chem. Soc. 2011, 133, 5652–5655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, P.; Wang, Y.; Zhang, J.; Zheng, J.; Zhang, P. Selective oxidation over a metalloporphyrinic metal-organic framework catalyst and insights into the mechanism of bicarbonate ion as co-catalyst. Chem. Eng. J. 2014, 257, 28–35. [Google Scholar] [CrossRef]
- Yang, X.-L.; Xie, M.-H.; Zou, C.; He, Y.; Chen, B.; O’Keeffe, M.; Wu, C.-D. Porous metalloporphyrinic frameworks constructed from metal 5,10,15,20-tetrakis(3,5-biscarboxylphenyl)porphyrin for highly efficient and selective catalytic oxidation of alkylbenzenes. J. Am. Chem. Soc. 2012, 134, 10638–10645. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Pan, M.; Hu, X.D.; Shao, W.H.; Li, J.; Zhang, F.X. Mn-III(porphyrin)-based porous coordination polymers: Synthesis, catalytic activities for the oxidation of ethylbenzene. Catal. Lett. 2016, 146, 1087–1098. [Google Scholar] [CrossRef]
- Xie, M.-H.; Yang, X.-L.; Wu, C.-D. A metalloporphyrin functionalized metal-organic framework for selective oxidization of styrene. Chem. Comm. 2011, 47, 5521–5523. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Cheng, Q.G.; Kim, C.; Gao, W.Y.; Wojtas, L.; Chen, Y.S.; Zaworotko, M.J.; Zhang, X.P.; Ma, S.Q. Crystal engineering of a microporous, catalytically active fcu topology mof using a custom-designed metalloporphyrin linker. Angew. Chem. Int. Ed. 2012, 51, 10082–10085. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Chrzanowski, M.; Wojtas, L.; Chen, Y.S.; Ma, S.Q. Formation of a metalloporphyrin-based nanoreactor by postsynthetic metalion exchange of a polyhedral-cage containing a metalmetalloporphyrin framework. Chem. Eur. J. 2013, 19, 3297–3301. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhang, T.; Xie, M.-H.; Yan, L.; Kong, G.-Q.; Yang, X.-L.; Ma, A.; Wu, C.-D. Four metalloporphyrinic frameworks as heterogeneous catalysts for selective oxidation and aldol reaction. Inorg. Chem. 2013, 52, 3620–3626. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.-H.; Yang, X.-L.; He, Y.; Zhang, J.; Chen, B.; Wu, C.-D. Highly efficient C-H oxidative activation by a porous Mn-III-porphyrin metal-organic framework under mild conditions. Chem. Eur. J. 2013, 19, 14316–14321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, P.; Wang, Y.; Zhang, J.; Zhang, P. An Efficient catalyst based on a metal metalloporphyrinic framework for highly selective oxidation. Catal. Lett. 2015, 145, 589–595. [Google Scholar] [CrossRef]
- Li, C.; Qiu, W.; Long, W.; Deng, F.; Bai, G.; Zhang, G.; Zi, X.; He, H. Synthesis of porphyrin@MOFs type catalysts through “one-pot” self-assembly. J. Mol. Catal. A Chem. 2014, 393, 166–170. [Google Scholar] [CrossRef]
- Chen, Y.; Hoang, T.; Ma, S.Q. Biomimetic catalysis of a porous iron-based metal-metalloporphyrin framework. Inorg. Chem. 2012, 51, 12600–12602. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Gu, Z.-Y.; Li, J.-R.; Jiang, H.-L.; Wei, Z.; Zhou, H.-C. Zirconium-metalloporphyrin PCN-222: Mesoporous metal-organic frameworks with ultrahigh stability as biomimetic catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, D.; Traylor, T.G.; Xie, L.Y. Polyhaloporphyrins: Unusual ligands for metals and metal-catalyzed oxidations. Acc. Chem. Res. 1997, 30, 251–259. [Google Scholar] [CrossRef]
- Wang, X.S.; Meng, L.; Cheng, Q.G.; Kim, C.; Wojtas, L.; Chrzanowski, M.; Chen, Y.S.; Zhang, X.P.; Mat, S.Q. Three-dimensional porous metal-metalloporphyrin framework consisting of nanoscopic polyhedral cages. J. Am. Chem. Soc. 2011, 133, 16322–16325. [Google Scholar] [CrossRef] [PubMed]



















| MOF a | Catalyzed Reaction (s) | Substrate (s) | Ref. |
|---|---|---|---|
| [(Mn(TpCPP)Mn1.5)(C3H7NO)]·5C3H7NO (PIZA-3) | Oxidation of linear and cyclic alkanes | cyclohexane, cycloheptane, hexane and heptane | [23] |
| Epoxidation of olefins | cyclopentene, cyclohexene, cyclooctene and limonene | ||
| ZnMn-RPM (Zn designates the metal in TpCPP while Mn designates the metal in F10DPyP) | Oxidation of cyclic alkanes | cyclohexane | [24] |
| Epoxidation of olefins | styrene | ||
| Fe-MMOF | Oxidation of alkanes | cyclohexane | [25] |
| Epoxidation of olefins | cyclooctene,cyclohexene, styrene, hex-1-ene, oct-1-ene, dodec-1-ene, 1H-indene, vinyl acetate, methyl acrylate | ||
| Oxidation of alcohols | cyclohexanol, benzyl alcohol, octan-2-ol, pentan-1-ol | ||
| [Mn5Cl2(MnCl-OCPP)(DMF)4(H2O)4]·2DMF·8CH3COOH·14H2O (ZJU-18) | Oxidation of aromatic alkanes | ethylbenzene, diphenylmethane, 4-phenyl-ethylbenzene | [26] |
| [Mn5Cl2(Ni-OCPP)(H2O)8]·7DMF·6CH3COOH·11H2O (ZJU-19) | ethylbenzene | ||
| [Cd5Cl2(MnCl-OCPP)(H2O)6]·13DMF·2CH3COOH·9H2O (ZJU-20) | |||
| MnIII(F5CPP)–MnII | Oxidation of aromatic alkanes | ethylbenzene | [27] |
| MnIII(F5CPP)–CoII | |||
| MnIII(F5CPP)–NiII | |||
| MnIII(F10CPP)–MnII | |||
| MnIII(F10CPP)-CoII | |||
| MnIII(F10CPP)-NiII | |||
| [Cd1.25(Pd−H1.5TpCPP)-(H2O)]·2DMF | Oxidation of alkenes | styrene | [28] |
| [Co2(μ2-H2O)(H2O)4](Co-DCDBP) (MMPF-3) | Epoxidation of olefins | trans-stilbene | [29] |
| [Cd8(Cd-OCPP)3][(H3O)8] (MMPF-5) | Epoxidation of olefins | trans-stilbene | [30] |
| [(CH3)2NH2][Zn2(HCOO)2(MnIII-TpCPP)]·5DMF·2H2O | Epoxidation of olefins | styrene, cyclopentene, cyclohexene, cyclooctene, terminal linear alkenes (hex-1-ene, oct-1-ene, dodec-1-ene), stilbene, and some modified stilbenes and styrenes | [31] |
| [(CH3)2NH2][Cd2(HCOO)2(MnIII-TpCPP)]·5DMF·3H2O | |||
| [Zn2(HCOO)(FeIII(H2O)-TpCPP)]·3DMF·H2O | styrene | ||
| [Cd3(H2O)6(μ2-O)(FeIII-HTpCPP)2]·5DMF | styrene | ||
| [Zn2(MnOH-TpCPP)(DPNI)]·0.5DMF·EtOH·5.5H2O (CZJ-1) | Epoxidation of olefins | styrene | [32] |
| MMPF | Epoxidation of olefins | cyclohexene, cyclooctene, hex-1-ene, oct-1-ene, dodec-1-ene, styrene, trans-stilbene | [33] |
| MnTNPP@MOF | Oxidation of alcohols | 3,5-di-tert-butylcathecol | [34] |
| Zr6O8(CO2)8(H2O)8-[FeCl(TpCPP)] (MMPF-6) | Oxidation of alcohols | 1,2,3-trihydroxybenzene | [35] |
| Zr6(OH)8-FeTpCPP (PCN-222 (Fe)) | Oxidation of alcohols | 1,2,3-trihydroxybenzene, 3,3′,5,5′-tetramethylbenzidine, o-phenylenediamine | [36] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, C.F.; Simões, M.M.Q.; Tomé, J.P.C.; Almeida Paz, F.A. Porphyrin-Based Metal-Organic Frameworks as Heterogeneous Catalysts in Oxidation Reactions. Molecules 2016, 21, 1348. https://doi.org/10.3390/molecules21101348
Pereira CF, Simões MMQ, Tomé JPC, Almeida Paz FA. Porphyrin-Based Metal-Organic Frameworks as Heterogeneous Catalysts in Oxidation Reactions. Molecules. 2016; 21(10):1348. https://doi.org/10.3390/molecules21101348
Chicago/Turabian StylePereira, Carla F., Mário M. Q. Simões, João P. C. Tomé, and Filipe A. Almeida Paz. 2016. "Porphyrin-Based Metal-Organic Frameworks as Heterogeneous Catalysts in Oxidation Reactions" Molecules 21, no. 10: 1348. https://doi.org/10.3390/molecules21101348
APA StylePereira, C. F., Simões, M. M. Q., Tomé, J. P. C., & Almeida Paz, F. A. (2016). Porphyrin-Based Metal-Organic Frameworks as Heterogeneous Catalysts in Oxidation Reactions. Molecules, 21(10), 1348. https://doi.org/10.3390/molecules21101348









