Abstract
The tropane and granatane alkaloids belong to the larger pyrroline and piperidine classes of plant alkaloids, respectively. Their core structures share common moieties and their scattered distribution among angiosperms suggest that their biosynthesis may share common ancestry in some orders, while they may be independently derived in others. Tropane and granatane alkaloid diversity arises from the myriad modifications occurring to their core ring structures. Throughout much of human history, humans have cultivated tropane- and granatane-producing plants for their medicinal properties. This manuscript will discuss the diversity of their biological and ecological roles as well as what is known about the structural genes and enzymes responsible for their biosynthesis. In addition, modern approaches to producing some pharmaceutically important tropanes via metabolic engineering endeavors are discussed.
1. Introduction
Plants are sessile organisms and thus evolved natural products or “specialized metabolites” as a chemical response to both biotic and abiotic forces. Specialized metabolites are used by plants to defend themselves and communicate with other plants and organisms in their environments. Whilst the chemical diversity of plant specialized metabolites is vast, with total numbers thought to exceed over 200,000 structures, common themes of structure and function are the result of repeated and convergent evolution of both their biosynthesis and biological roles [1]. Moreover, the chemical structures and underlying biosynthetic enzymes of specialized metabolites serve as inspiration to medicinal and natural product chemists.
Many specialized metabolites are pharmacologically active and have been used by humans for therapeutic and recreational purposes since the beginning of recorded history. In particular alkaloids, pharmacologically active cyclic nitrogen containing metabolites derived from amino acids, are known for their pharmacological effects and frequently serve as the starting point for drug development [2]. Some of the oldest domesticated medicinal plants have been those that produce alkaloids. For example, Erythroxylum coca, a species notable for the production of the tropane alkaloid cocaine (1), was used in Peruvian households at least 8000 years ago [3]. Similarly, pomegranate (Punica granatum) has a history of cultivation that goes back at least 10,000 years in Egypt and is well-known for the production of granatane alkaloids [4].
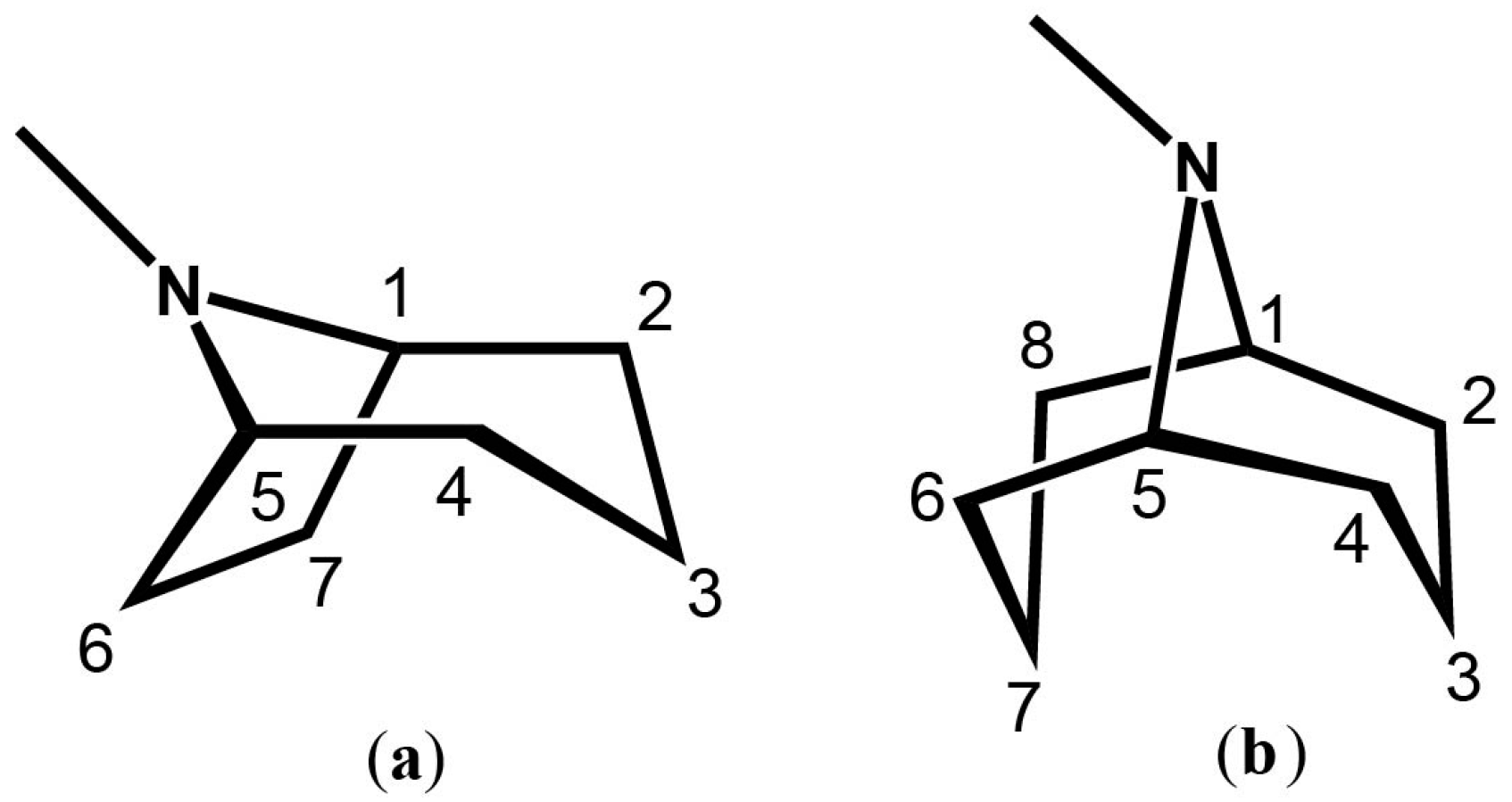
Tropane (TA) and granatane (GA) alkaloids are structural homologues, sharing similar chemical compositions and core scaffolds. Despite their similarities, TAs and GAs show different distribution patterns across the plant kingdom. The N-methyl-8-azabicyclo[3.2.1]-octane core structure of TAs is found in over 200 alkaloids [2,5,6] (Figure 1). In contrast, N-methyl-9-azabicyclo[3.3.1]-nonane, the core scaffold of GAs, appears in considerably fewer alkaloid metabolites (Figure 1). The bicyclic core structures of TAs and GAs differ by only one carbon atom, yet this difference alters the conformational preferences of each of the core skeletons [7]. Furthermore, the presence or absence of a single carbon atom in the core rings of TAs and GAs alters their chemical and pharmacological activities.
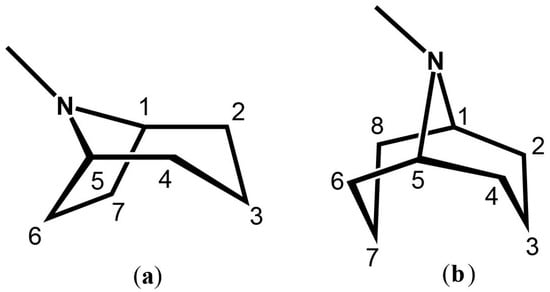
Figure 1.
(a) The tropane core skeleton; (b) The bicyclic granatane core skeleton. Both structures depict their chemically accepted carbon numbering.
1.1. Similarities and Differences in Medicinal Properties
Despite their structural similarities TAs and GAs have distinct medicinal properties. TAs have been considered panaceas throughout recorded history, especially because of their anticholinergic properties. Anticholinergics are a class of compounds used as drugs to block the action of the acetylcholine neurotransmitter to treat motion sickness and diseases such as Alzheimer’s and Parkinson’s [8]. The methylated nitrogen in the core ring of cocaine (1) and other TAs serves as a structural analog of acetylcholine. TAs have been observed to attach to and inhibit muscarinic acetylcholine receptors [9]. TAs found in the Solanaceae are well known for both their anticholinergic and antispasmodic properties that affect the parasympathetic nervous system [10,11,12]. These plants have been used for pain relief, anesthesia, and as a treatment for drug addiction [10]. Daturae Flos, the dried flowers of Datura metel also known as “yangjinhua” in China, has been utilized and recorded in the Chinese Pharmacopoeia as an anesthetic and was prescribed to treat cough, asthma and convulsions [13]. Przewalkia tangutica is a rare medicinal solanaceous plant found in the Tibetan Plateau of China in which the roots, seeds and entire vegetative tissues are utilized [14]. P. tangutica contains several biologically active TAs including anisodamine (2), scopolamine (3) and atropine (the racemic mixture of hyoscyamine (4). The TAs present in P. tangutica are associated with many biological activities including analgesic, spasm modulation, pesticidal, and anti-inflammatory effects [14]. Hyoscyamus niger, also known as henbane, has been utilized in Chinese traditional therapy as well as in Tibetan medicine [15]. H. niger has been used as a sedative and sleep agent [16]. Hyoscyamine (4) and scopolamine (3) are the dominant TAs of H. niger and both metabolites can cross the blood-brain barrier to effect the central nervous system [17]. Scopolamine (3) has more potent pharmaceutical activity when compared to hyoscyamine (4) and exhibits relatively fewer side effects, however the scopolamine (3) content of solanaceous plants is usually much lower than the hyoscyamine (4) content [11]. Because of this, there is an ongoing effort to fully understand the biosynthesis of scopolamine (3) and other TAs within the Solanaceae (see Section 4).
The narcotic properties of cocaine (1), a TA from the non-solanaceous genus Erythroxylum, can be attributed to unique modifications of the TA core scaffold that are not present in TAs from solanaceous plants. The carboxylic acid methyl ester present at the C2 position is responsible for the binding of cocaine (1) to the dopamine transporter [18]. Cocaine (1) has also been reported to block the reuptake of nor-epinephrine, serotonin (5-HT receptor) and dopamine (D-A receptor) by the binding of the aromatic ring present at the 3β position of the molecule to specific sites in these receptors, affecting the normal physiology of the central nervous system [19]. This stereospecific conformation is dominant in TAs found in the Erythroxylaceae, but is only a minor constituent in solanaceous plants.
While no direct studies regarding the anticholinergic effects of GAs have been reported, a computational and NMR based study comparing the structures of TAs and GAs revealed that GAs adopt an N-axial form that is similar to many known TAs [20]. The N-methyl group in the axial conformation is thought to be the pharmacophore for TAs [21]. Additionally, the granatane ring system provides the semisynthetic intermediate for the potent antiemetic agents Dolasteron and Granisetron, which are serotonin 5-HT3 receptor agonists [22,23]. These compounds are used as medicines to prevent acute nausea in patients undergoing chemotherapy and radiotherapy for the treatment of cancer [24].
GAs and TAs have different physiological effects beyond the central nervous system. Unlike TAs, GAs exhibit anti-proliferative effects on hepatoma cells [24]. Specifically, murine hepatoma (BNL CL.2) and human hepatoma (HepG2) cell lines were cultured with various doses of crude GA-containing alkaloid fraction extracts from Sedum sarmentosum. Inhibition of excessive growth of tumor cells was observed, indicating that these compounds possess anti-cancer properties [25]. Other physiological effects that distinguish GAs from TAs include the use of GAs as an anti-worm treatment. Since the isolation of the first GAs, these compounds have been claimed to possess anti-worm (anthelminthic) capabilities, which were then studied in detail by scientists in the University of Amsterdam in 1956. These studies focused on deriving which GAs possessed the highest anthelminthic activity. The anthelminthic activity of synthetic granatane and its derivatives were measured in liver fluke. Their results rendered the highest anthelminthic activity to the compound isopelletierine [26]. These findings were later scientifically supported in 1963 with newer and better chemical methods [27]. Fascioliasis, a disease caused by liver fluke Fasciola hepatica, is common in cattle. Molluscidal activity in pomegranate bark extracts was effective in killing of Lymnaea acuminata, the vector for F. hepatica [28,29]. Beyond their medicinal properties, GAs also differ from TAs in that GAs have been found to be useful in the prevention of corrosion in the oil, gas and metal industries [30].
1.2. The Scattered Distribution of Tropanes and Granatanes amongst Angiosperms
Tropane alkaloids are commonly found in the genus Erythroxylum of the Erythroxylaceae family. The Erythroxylum genus includes at least 230 species that are distributed throughout the tropical regions of South and Central America [3,31,32]. Erythroxylum coca was one of the first domesticated plant species that provided nutritional, medicinal, and digestive properties to ancient civilizations by chewing the leaves of the plant [33]. Most of the cultivated coca used for cocaine (1) production comes from this species [34]. Albert Neimann first isolated cocaine (1) as a pure substance in 1860 [35] and its use exploded in popularity following an endorsement by Sigmund Freud [36]. The leaves of Erythroxylum novogranatense were also chewed by the elite class for their high content of methyl salicylate, which imparts a minty taste [37,38]. This species is known as “Colombian coca” and is found to be cultivated in the mountains of present day Colombia. “Trujillo coca” (E. novogranatense var. truxillense) is a cultivar that is grown in dry and arid regions. This species is also rich in methyl salicylate and contains other flavoring qualities that are still used in the production of Coca Cola, however today the extracts are decocainized [34].
Atropine, scopolamine (3), and hyoscyamine (4) are a few well-known TAs from the Solanaceae family. As discussed above, these compounds are commonly found in species such as Atropa belladonna, H. niger, and many members of the genus Datura. Scopolamine (3) was first isolated in 1888 from Scopolia japonica [39]. In medieval Europe, extracts from A. belladonna, were used as poisons, hallucinogens and aphrodisiacs [17]. Five to ten berries of A. belladonna could kill a person. The toxicity of the extracts has also been used on arrows to poison victims [40]. When extracts of A. belladonna are applied to the eyes, dilation of the pupils occurs [41]. For this reason, women used A. belladonna as a cosmetic drug during the Renaissance. Women of the 15th century who were devoted to witchcraft also exploited the psychoactive effects of A. belladonna [16]. Mucous membranes, such as those found in the walls of the oral cavity and the vulva, are readily susceptible to drug absorption. It is believed that the application of alkaloid-containing salves to the skin or vulva was achieved by the use of brooms. It gave users the feeling of being able to fly, feeding the folkloric associations of witches with brooms [17]. Atropine was first isolated in 1833 from A. belladonna [42,43]. The correct structure of atropine was obtained by Willstätter in 1889 after much deliberation and structural studies [17]. Leaves of D. metel from solanaceous plants were used as herbal cigarettes in the 19th and 20th centuries to treat patients with asthma or other respiratory conditions [17].
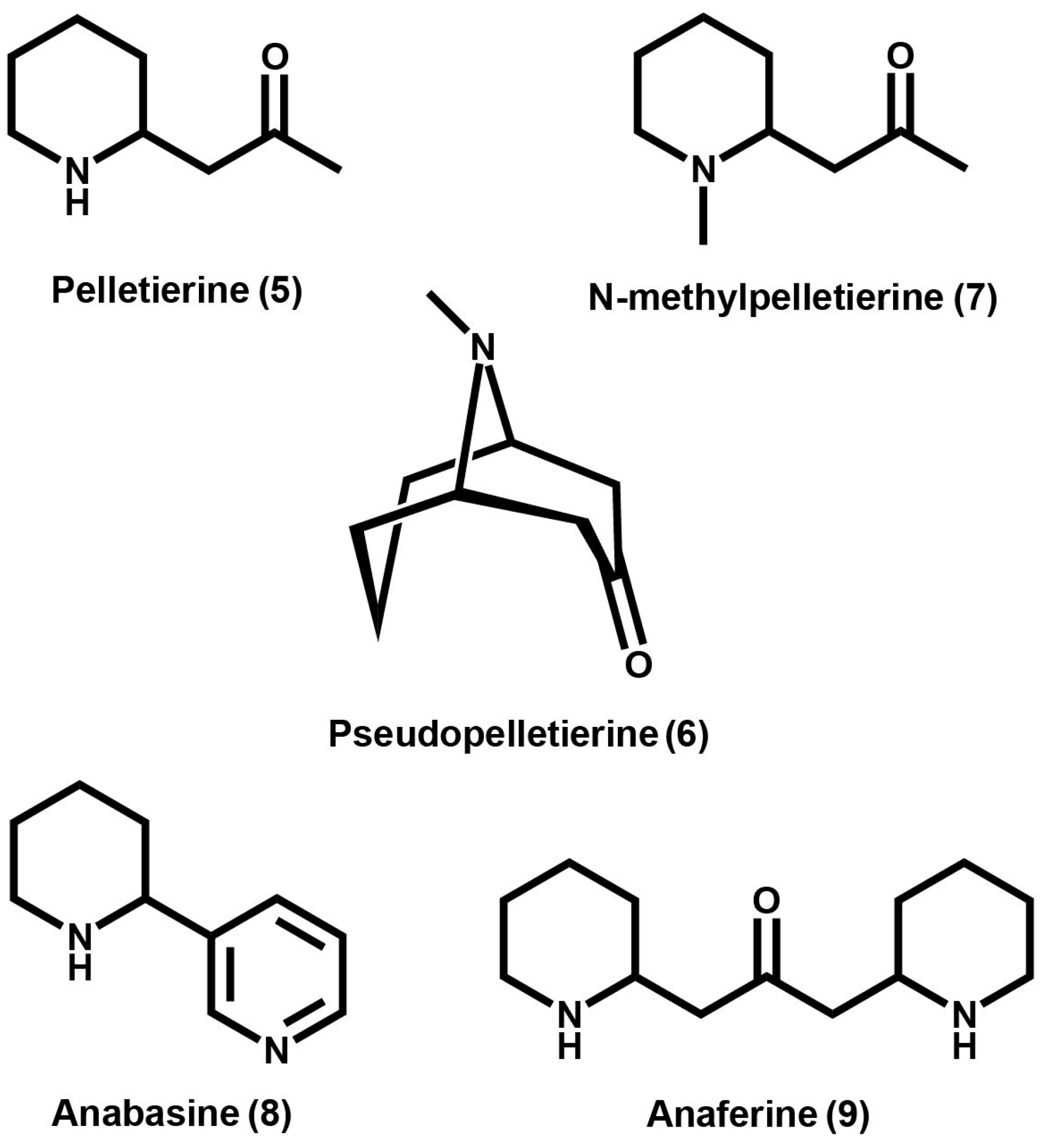
GAs include pelletierine (5), isopelletierine, pseudopelletierine (6), and N-methylpelletierine (7) and their derivatives anabasine (8) and anaferine (9) (Figure 2). GAs are predominantly found in P. granatum although they have been characterized in other species such as S. sarmentosum, and Withania somnifera [44,45,46,47]. The pomegranate tree is native to Iran, Afghanistan, Baluchistan, and Himalayas in Northern India. Pomegranate can also be found in the Mediterranean and Caucasus regions due to its ancient cultivation. Today, pomegranate is cultivated all over India, Southeast Asia, Malaysia, the East Indies, tropical Africa, and the United States [48]. In 1879, French chemists Tanret and Pelletier isolated a basic substance from the root bark of the pomegranate tree and characterized the salt [45]. As of this review, no studies regarding characterization of any structural genes or the enzymes responsible for the production of GAs has been reported. Interestingly, the isolation of pseudopelletierine (6) has been reported in the species Erythroxylum lucidum [49] suggesting that GAs and TAs may use similar biosynthetic machinery.
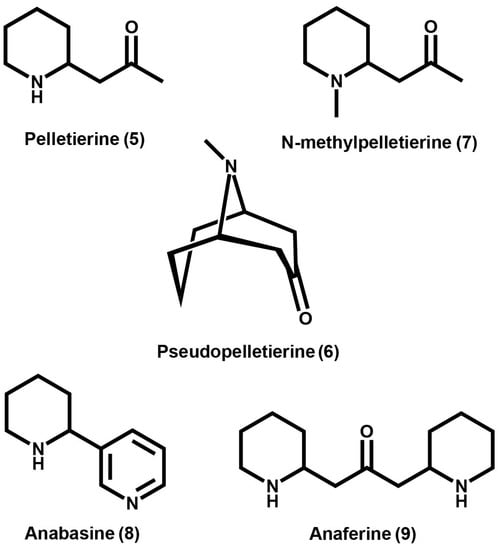
Figure 2.
Examples of granatane alkaloids and granatane alkaloid derivatives found among several plant species.
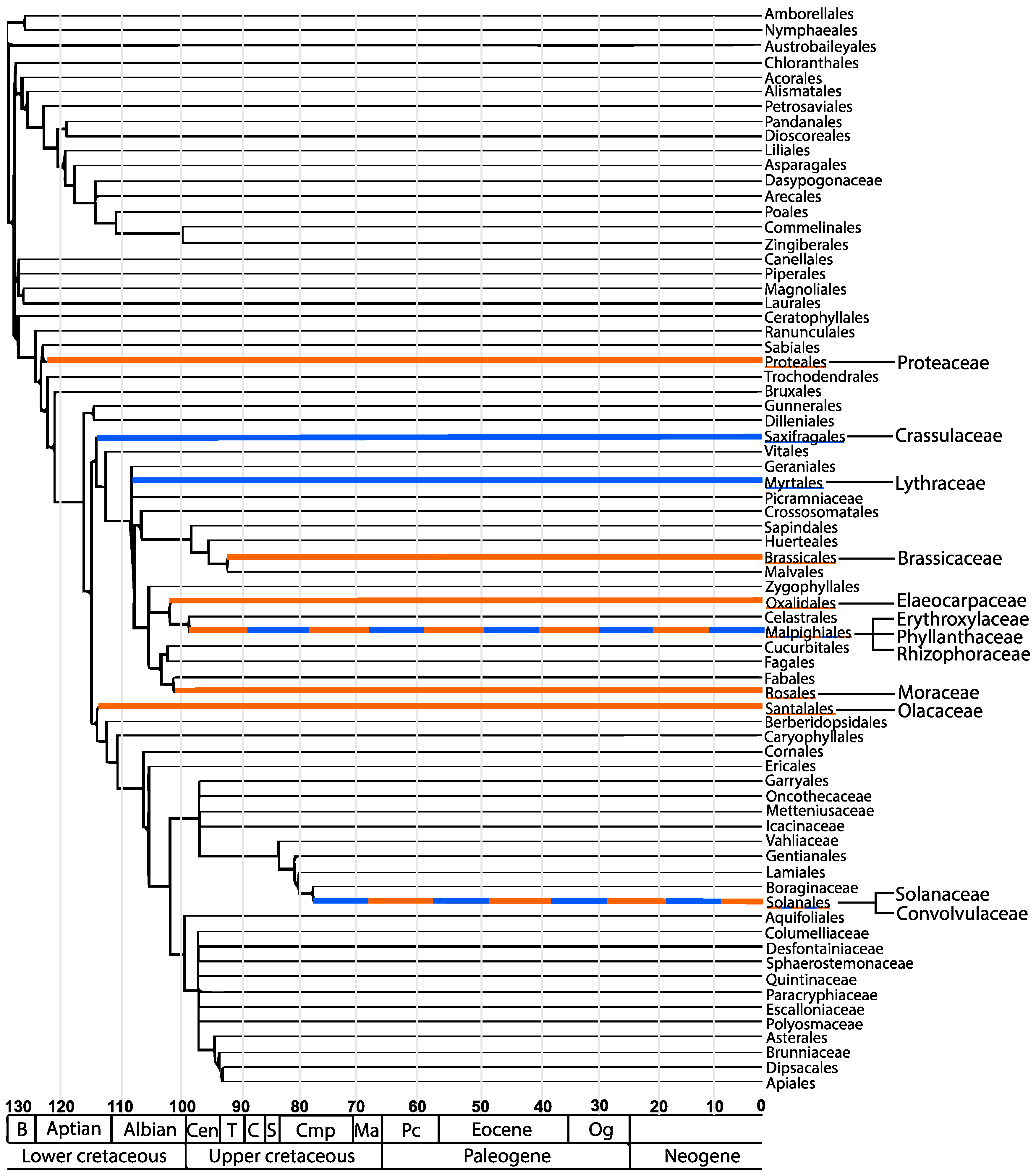
Scientific reports on the occurrence and distribution of TAs from angiosperm families other than the Solanaceae and Erythoxylaceae are few. When viewed against a phylogeny of angiosperms, the results reveal a scattered and non-contiguous distribution (Figure 3) [50,51]. For example, TAs have been reported in members of the family Proteaceae. Compounds found in the family Proteaceae include the pyranotropanes, strobamine and bellendine as well as the compounds ferruginine and ferugine [52]. In addition, a few members within the families Brassicaceae and Convolvulaceae have been found to produce calystegines, which are heavily hydroxylated forms of TAs [53]. The main TA producing species are scattered among four orders, which include the Solanales, Malpighiales, Proteales and Brassicales. In the case of GAs, P. granatum from the family Lythraceae is the main producing species. However, the appearance of GAs has also been reported in members of the Solanaceae, Crassulaceae, and Erythroxylaceae families. These families belong to the orders Myrtales, Solanales, Malpighiales, and Saxifragales. This distribution pattern raises the important question about the biosynthetic origin of the respective GA and TA pathway. Namely, have these biosynthetic pathways arisen from a common ancestor or have they arisen independently in several cases? Recent evidence in TA biosynthesis suggests that certain biosynthetic steps have multiple origins [5]. By extension, this could also mean that GA biosynthesis has arisen independently more than once.
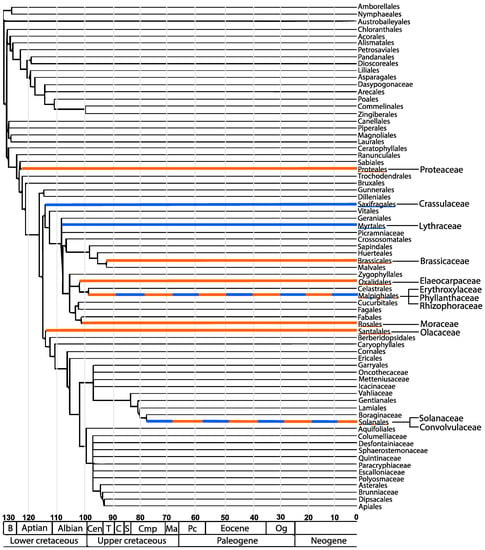
Figure 3.
The diversity and similarities of tropane and granatane producing angiosperms. The orders tropane alkaloids belong to are highlighted in orange. The orders granatane alkaloids belong to are highlighted in blue. Shared orders are represented by both colors. The plant families that are known to produce tropane and granatane alkaloids are branched off from the orders. The scale at the bottom represents millions of years. Modification of the phylogenetic tree published in [54], republished with permission with the Botanical Society of America.
Examples of scattered distributions of alkaloid classes can be observed throughout the plant kingdom. For example, the legumes (Fabaceae) contain several alkaloid secondary metabolites that are not found in all members within the family [55]. In some cases, de novo evolution of the pathways have been observed. In the case of indolizidine alkaloids, the source of the scattered appearance of alkaloids is theorized to occur due to horizontal gene transfer from alkaloid producing endophytic fungi.
1.3. Biosynthesis of TAs and GAs
Diversity among tropane and granatane alkaloids can also be seen at the biochemical level. Most data available regarding enzymes involved in TA biosynthesis comes from species within the Solanaceae family. Despite recent advancements, some critical steps in TA biosynthesis remain ambiguous. Additionally, recent advances in high throughput sequencing technologies, plant genomics, and biochemical methods have aided the progress of elucidating TA biosynthesis in other non-model species, specifically within the Erythroxylaceae. Lastly, even though the biosynthesis of GAs is predicted to be similar to TAs, plausibly a whole new set of enzymes were recruited for the biosynthesis of GAs. This would mean that untapped gene and enzyme diversity is present in the GA and TA biosynthetic pathways. It is therefore important to elucidate the biosynthesis of these alkaloids to help further understand specific parts of their mechanisms and their metabolic processes.
2. Tropane Alkaloid Biosynthesis
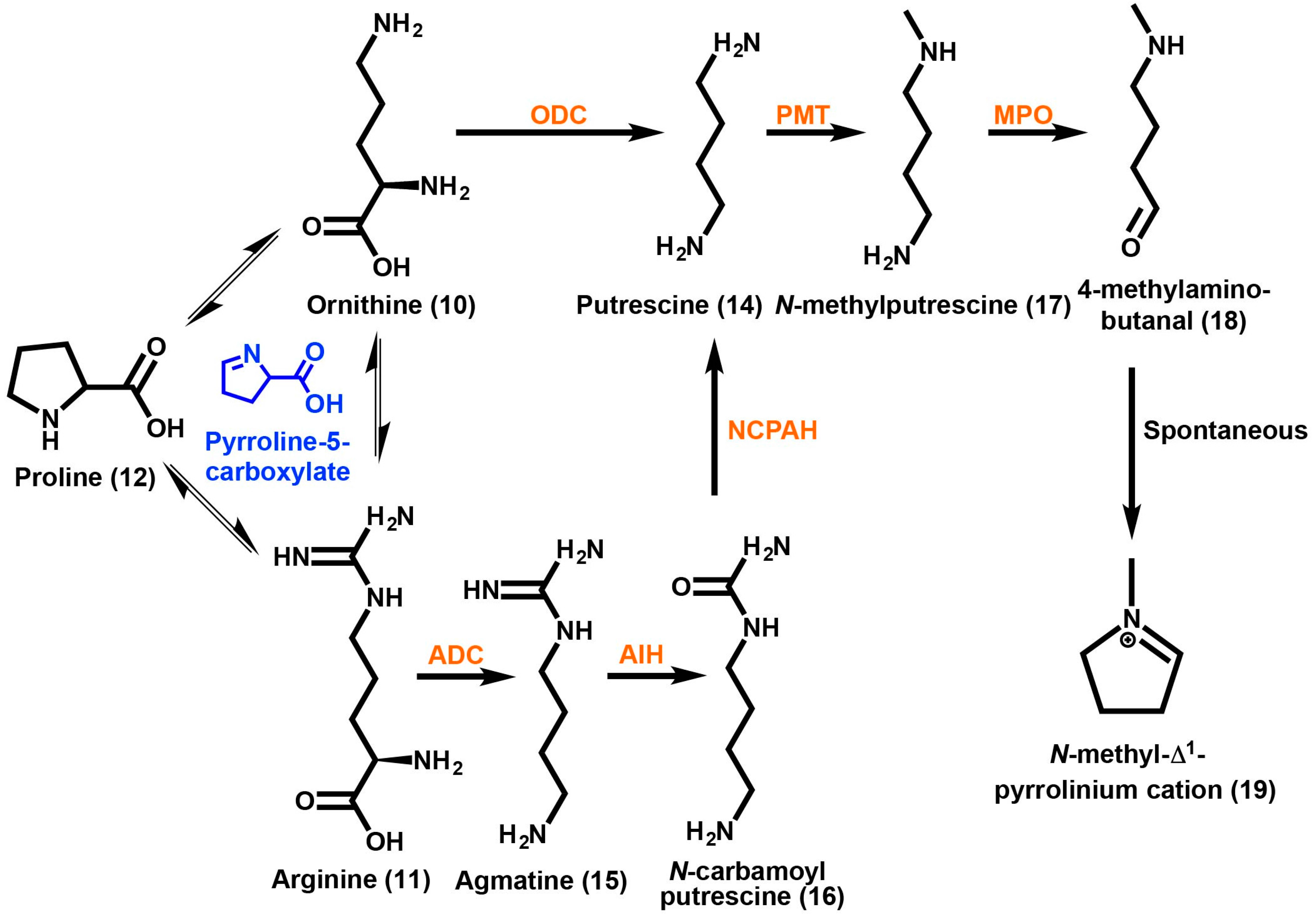
Overall, the process of producing either TAs or GAs in plants begins with the diversion of specific amino acids from primary metabolism into the formation of an initial nitrogen-containing ringed compound. This heterocycle will in most cases proceed on to form a bicyclic structure in which the core skeletons are created (Figure 1). Further modifications will add diverse functional groups to the core structure yielding the final end product. Ornithine (10) and arginine (11) are the amino acids predicted to be the starting substrates of TA biosynthesis (Scheme 1) [56]. Feeding studies using 14C-proline into the roots of A. belladonna also suggest that proline (12) may be a starting amino acid for incorporation into the tropane moiety. Other studies using D. metel and D. stramonium have also shown the incorporation of proline (12) into TA compounds, such as scopolamine (3) and tropine (13) [57]. Biosynthetically each of these three amino acids can generate a pyrroline-5-carboxylate intermediate, which can be interconvertible between these amino acids [58]. Consequently, feeding studies of labeled amino acids are difficult to interpret without further enzymological data.
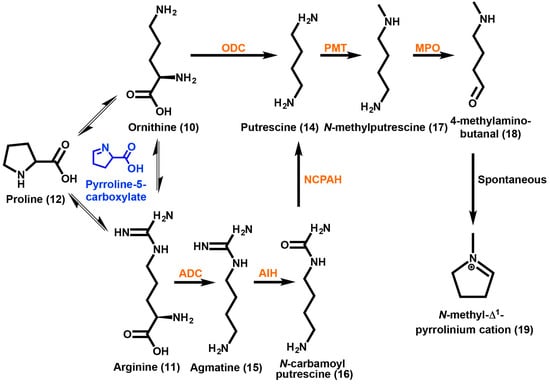
Scheme 1.
Tropane alkaloid biosynthesis up to the formation of the N-methyl-Δ1-pyrrolinium cation. Arginine, ornithine and proline are interconvertable, sharing the pyrroline-5-carboxylate intermediate (highlighted in blue). Putrescine can be formed directly via decarboxylation of ornithine or indirectly through arginine. Methylation of putrescine is followed by oxidation to yield 4-methylamino-butanal, which is then spontaneously cyclized to yield the N-methyl-Δ1-pyrrolinium cation. Enzymes are highlighted in orange and abbreviated as follows: ADC, arginine decarboxylase; AIH, agmatine imino hydrolase; NCPAH, N-carbamoylputrescine amino hydrolase; ODC, ornithine decarboxylase; PMT, putrescine N-methyl transferase; MPO, methyl putrescine oxidase.
A nonsymmetrical intermediate has been proposed for the production of the pyrrolidine ring, if the amino acid ornithine (10) is first methylated at the γ-N position. A proposed alternative route includes the decarboxylation of ornithine (10) to form the polyamine putrescine (14) as the first step [59,60]. Feeding studies of radioactively labeled ornithine-2-14C have shown that several Datura species incorporate a nonsymmetrical intermediate [59]. However, a symmetrical intermediate has been reported for Nicotiana, Erythroxylum, and Hyoscyamus species [61]. Symmetrical incorporation showed activity at positions C1 and C5 of the tropane ring and has been reported for Nicotiana, E. coca, and Hyoscyamus albus [59,60]. A one-step enzymatic approach is possible to reach a symmetrical intermediate by converting ornithine (10) into putrescine (14) catalyzed by ornithine decarboxylase (ODC) [62]. Another route to putrescine (14) can be taken by starting with the amino acid arginine (11). The decarboxylation of arginine (11) into agmatine (15) is catalyzed by arginine decarboxylase (ADC). Agmatine (15) can then be converted into N-carbamoyl putrescine (16) via agmatine imino hydrolase (AIH). The enzyme N-carbamoyl putrescine amido hydrolase (NCPAH) then yields putrescine (14). Both the ADC and ODC directed pathways have different and diverse outcomes in regards to primary and secondary metabolism. Putrescine (14) that was produced by ODC is important for the supply of polyamines for primary metabolic processes such as cellular differentiation, development and division [63]. On the other hand, putrescine (14) that was produced by ADC in the Solanaceae is thought to be required for environmental stress related responses.
The formation of N-methylputrescine (17) catalyzed by putrescine N-methyltransferase (PMT) is considered the first rate-limiting step in the TA biosynthetic pathway [64]. PMT is an S-adenosylmethionine (SAM)-dependent methyltransferase that attaches a methyl group to the nitrogen atom that ultimately appears in the tropane skeleton. The first PMT sequence to be isolated from plants came from tobacco (Nicotiana tabacum) [65]. Since then, many other PMT related sequences have been isolated and characterized from other pyrrolidine alkaloid producing plant species. PMT belongs to a large family of enzymes that are involved in polyamine production. These primary metabolites play an important role in stress physiology, senescence, and morphogenesis [66]. Several lines of evidence suggest that PMT function has evolved from an ancestral spermidine or spermine synthases [67]. This would suggest that an enzyme from primary metabolism was recruited for the production of secondary metabolites. Localization of the PMT protein is root specific for both nicotine and tropane producing solanaceous plants [68,69,70]. While N-methylputrescine (17) is the predominant intermediate for solanaceous TAs, at least one alternative has been suggested. [6-14C]-1,5,10-triazadecane fed to Nicotiana glutinosa plants revealed that N-methylspermidine can also be incorporated into the pyrrolidine ring of nicotine [71].
The next step in TA biosynthesis is the formation of 4-methylamino-butanal (18) from N-methylputrescine (17) catalyzed by methylputrescine oxidase (MPO). This enzyme coexists with a class of copper-dependent diamine oxidases that uses copper as a cofactor to oxidize a conserved tyrosine residue into a topaquinone, essential for enzyme catalysis [72]. MPO was first characterized from the species N. tabacum [73,74]. Although an MPO like gene has yet to be discovered in E. coca, the corresponding activity from intact plants fed 4-monodeuterated N-methylputrescine shows the same enantioselectivity as solanaceous plants that produce either nicotine or TAs [75]. Using 13C fractionation techniques, researchers have found that both nicotine and hyoscyamine (4) share the same biosynthetic pathway, at least up to the N-methyl-Δ1-pyrrolinium cation (19) [76]. Subsequently, a hypothesis has been proposed that a metabolic channel prevails in which a multi-enzyme complex is active. The product of MPO, 4-methylamino-butanal (18), spontaneously cyclizes to form the N-methyl-Δ1-pyrrolinium cation (19) [59]. Feeding studies using ornithine-2-14C detected labeled 4-methylamino-butanal in D. stramonium plants [59]. The N-methyl-Δ1-pyrrolinium cation (19) serves as the first ring in the bicyclic tropane skeleton.
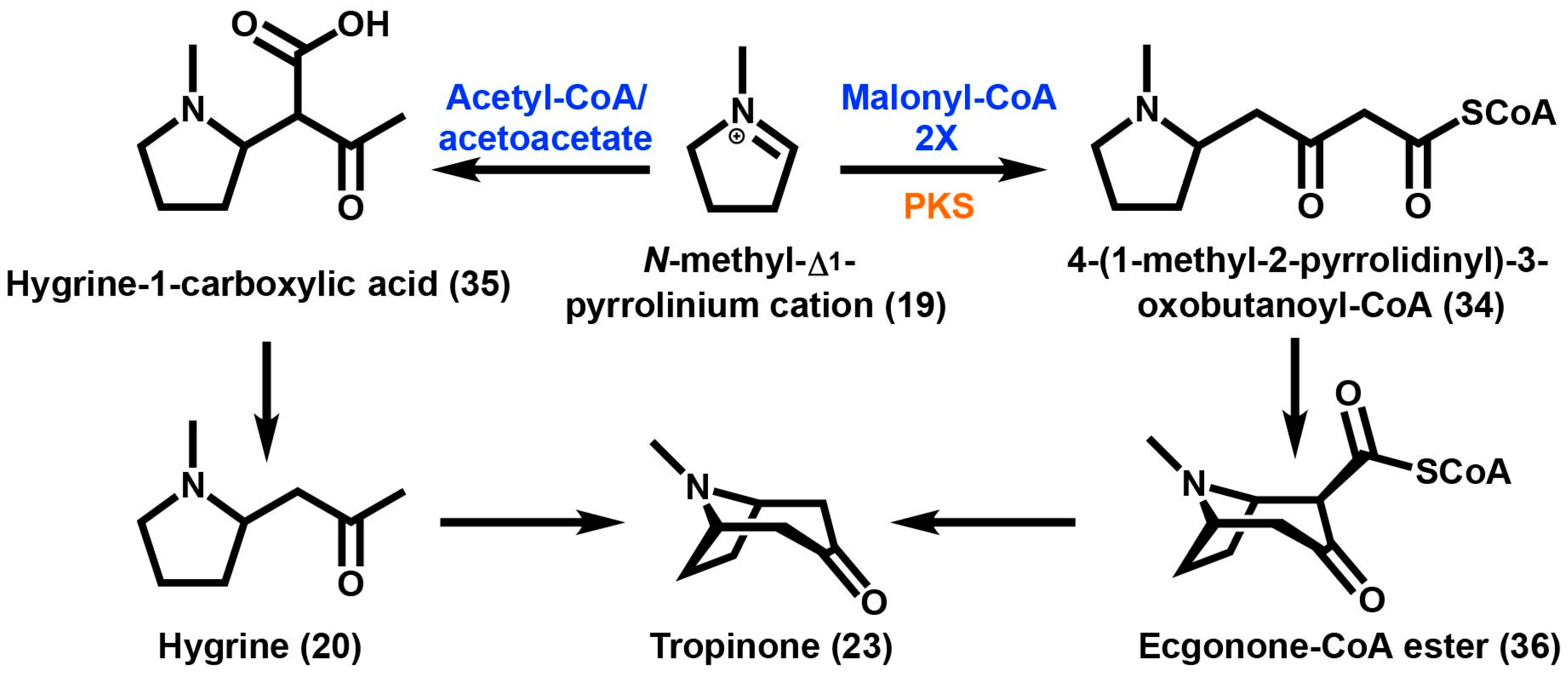
While the biochemical role of PMTs and MPOs are generally accepted steps leading to the first ring closure of tropane intermediates in the Solanaceae, the enzymatic basis of the second ring closure is controversial. For many years, the compound hygrine (20), (R)-1-(1-methylpyrrolidin-2-yl)-propan-2-one was thought to be a direct intermediate based on feeding studies, however more recent studies have demonstrated that previous results are likely experimental artifacts [77,78,79]. In solanaceous plants the best incorporation into the second ring was achieved from racemic ethyl [2,3-13C2]-4-(N-methyl-2-pyrrolidinyl)-3-oxobutanoate [77,80], a polyketide based molecule (Scheme 2). Further evidence for the involvement of a polyketide was demonstrated by the feeding of methyl (RS)-[1,2-13C2,1-14C]-4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoate to the leaves of E. coca [81]. This strongly implicates the involvement of acetate-derived metabolites (e.g., polyketides) during the formation of the second ring in TAs. There are limited types of enzymes capable of catalyzing this type of condensation reaction. In the case of benzylisoquinoline alkaloid biosynthesis a Pictet–Spenglerase enzyme was shown to condense dopamine with 3,4-dihydroxyphenylacetaldehyde [82]. However, based on more recent isotope and radiolabeled feeding studies it is hypothesized that a type III polyketide synthase (PKS) reaction is involved in condensing acetate units onto the N-methyl-Δ1-pyrrolinium cation (19) leading to the second ring closure of TA biosynthesis [77,80,83]. Type III PKSs are a family of enzymes known to catalyze the iterative decarboxylative condensation of malonyl-CoA onto CoA-tethered substrates. Type III PKSs are promiscuous enzymes that have a broad tolerance for diverse substrates and are able to catalyze multiple reactions [84,85]. Type III PKSs that use cyclic nitrogen-containing substrates have been previously characterized for their roles in alkaloid production [86,87,88]. However, unlike these previous studies the predicted substrate in TA metabolism, N-methyl-Δ1-pyrrolinium cation (19) is charged and lacks a CoA thioester.
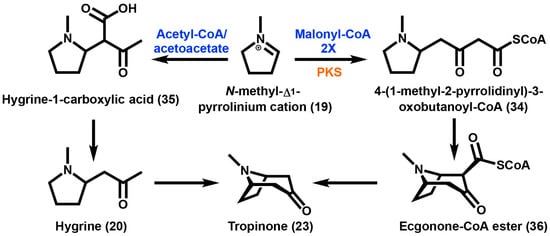
Scheme 2.
Tropinone formation from the N-methyl-Δ1-pyrrolinium cation in which there are two possibilities for the condensation of the tropane ring. Acetyl-CoA is utilized via acetoacetate to yield hygrine-1-carboxylic acid. On the other hand, two successive decarboxylative condensations of malonyl-CoA by PKS (polyketide synthase) yields 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoyl-CoA. Enzymes are highlighted in orange and substrates are highlighted in blue.
Spontaneous decarboxylation at the C2 position following ring closure can be prevented by the formation of the methyl ester. This would explain the presence of the carbomethoxy group found in cocaine (1) as well as other tropane alkaloids produced by plants found in the Erythroxylaceae. The resulting compound methylecgonone (21), contains a keto function at the C3 position and a carbomethoxy group at the C2 position [5]. Reduction at C3 is necessary for ester formation to occur.
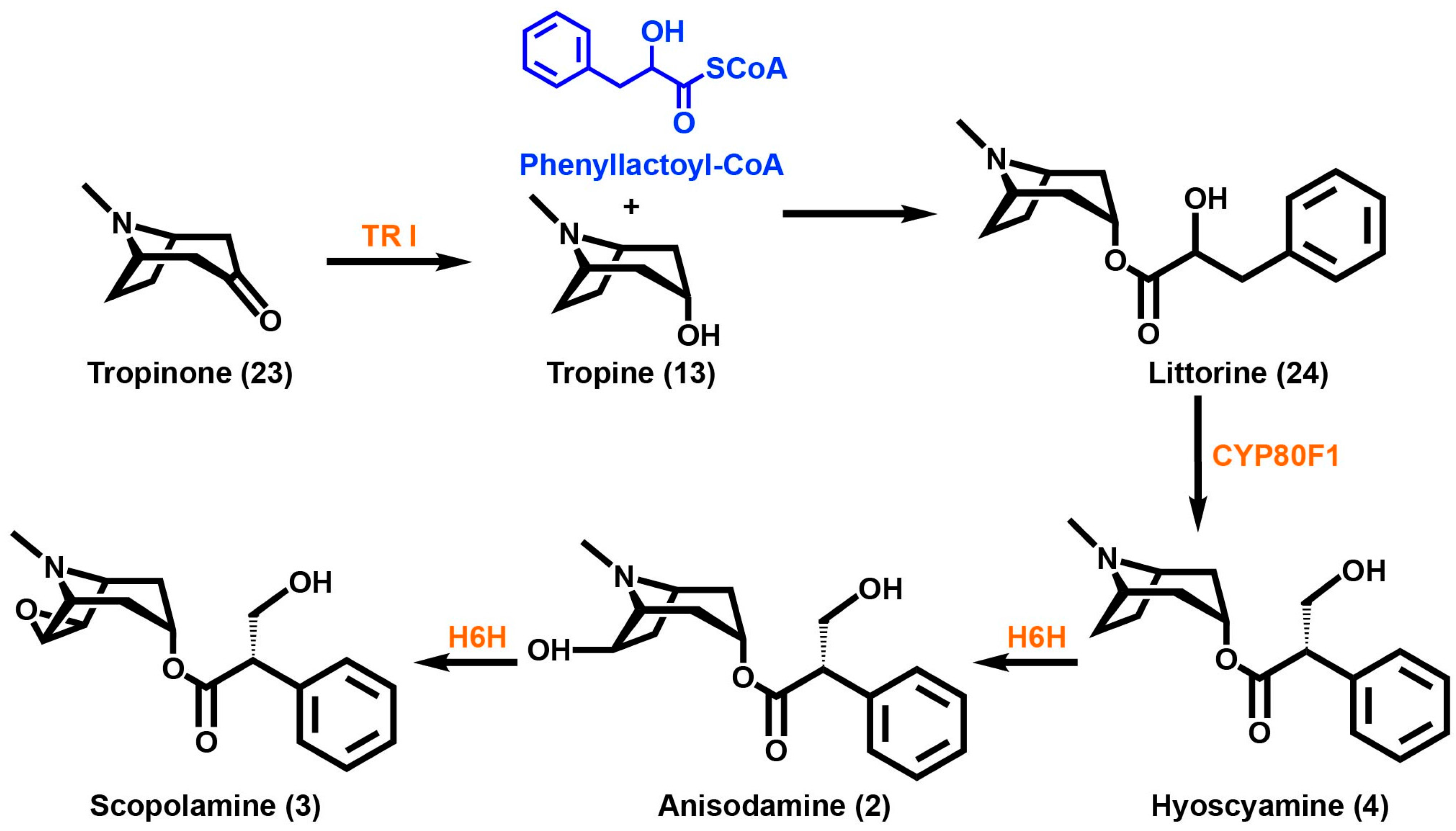
For the members of the Solanaceae, tropinone reductase (TR) enzymes catalyze the reduction of keto groups in the tropane ring. These enzymes are part of the short chain dehydrogenase/reductase (SDR) family that catalyzes NAD(P)(H)-dependent monomeric oxidoreductase reactions [89]. Its activity controls the metabolic flux towards TA biosynthesis downstream [90]. In solanaceous plants, two types of tropinone reductases exist, tropinone reductase I (TR I) and tropinone reductase II (TR II). They share a common tertiary “Rossman” fold structure, a conserved motif that consists of two pairs of α-helices and six parallel β-sheets, a catalytic active site with the motif YxxxK, and a dinucleotide cofactor-binding motif [91]. These enzymes share more than 50% of amino acid sequence similarity and are also assumed to have evolved from a common ancestor [90]. As little as five amino acid differences are needed to change the stereochemistry of the product [92]. TR I only converts the 3-keto function to a product that has a 3α-configuration (Scheme 3). This produces tropine (13) (3α-tropanol), which serves as a precursor for a wide range of esterified TAs [5]. On the contrary, TR II produces an alcohol solely with a 3β-configuration called pseudotropine (3β-tropanol). This is then converted to different nonesterified TAs called calystegines. A gene duplication event is attributed to these two different TRs in the Solanaceae family [93].
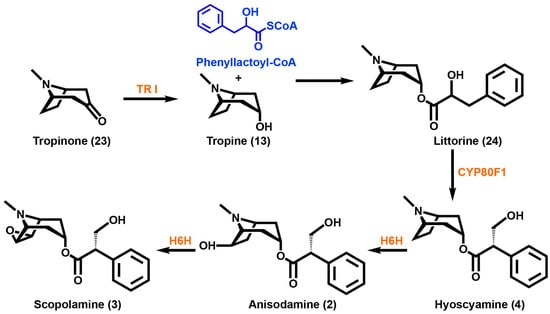
Scheme 3.
Tropinone is converted into tropine via TRI, tropinone reductase I. Tropine utilizes phenyllactoyl-CoA to yield littorine, which then uses a cytochrome p450 enzyme to form hyoscyamine. Scopolamine is epoxidized by the enzyme H6H, 6β-hydroxy hyoscyamine epoxidase, in a two-step process. Enzymes are highlighted in orange and substrates are highlighted in blue.
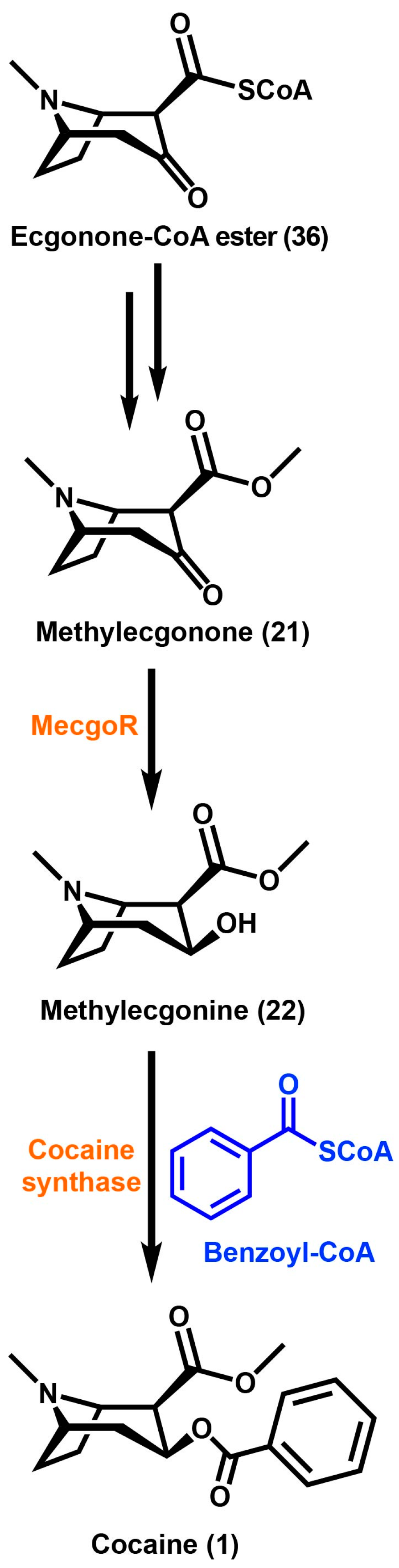
However in E. coca, a very different type of reductase enzyme was found for the reduction of the 3-keto function of methylecgonone (21) (Scheme 4). This is the first evidence of a polyphyletic origin for TA biosynthesis in plants. Methylecgonone reductase (MecgoR) was purified from crude protein extracts from coca leaves using classical biochemical techniques [5]. This enzyme was found to be very different than the TR enzymes that catalyzed the reduction reaction of the 3-keto function in solanaceous plants. MecgoR shares an overall identity of less than 10% at the amino acid level when compared to any TRs. MecgoR belongs to an aldo-keto reductase (AKR) superfamily of enzymes. AKRs that have been characterized so far share a common α/β-barrel motif, using either NADH or NADPH as a cofactor [94]. The MecgoR protein is localized in the palisade parenchyma of young developing leaves. This is in contrast to the localization of roots in TRs of solanaceous plants. This enzyme is also similar to other enzymes of alkaloid metabolism such as codeinone reductase and an enzyme of flavonoid biosynthesis, chalcone reductase. The stereospecific enzyme MecgoR catalyzes the conversion of methylecgonone (21) to the 3β-hydroxy-containing compound methylecgonine (22). MecgoR can also use tropinone (23) as a substrate but this only produces pseudotropine, which is consistent in E. coca with the presence of only 3β-hydroxy esters. Common TAs are esterified with aromatic or aliphatic acids, with the stereochemistry of the hydroxyl group dependent on the reductase used.
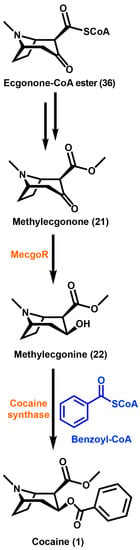
Scheme 4.
Ecgonone-CoA ester undergoes a multi-step process to yield methylecgonone, which is reduced by methylecgonne reductase, MecgoR to produce methylecgonine. Cocaine is then formed by the acylation of methylecgonine with benzoyl-CoA. Enzymes are highlighted in orange and substrates are highlighted in blue.
The benzoic ester of methylecgonine (22) is cocaine (1). Methylecgonine (22) is a molecule that has little physiological activity until it is converted into cocaine (1) [95]. There has been a prediction that an acyltransferase in E. coca utilizes benzoyl-CoA as the activated acid. This prediction was based on feeding studies using trans-[3-13C,14C]-cinnamic acid and the N-acetylcysteamine thioester of [3-13C,14C]-trans-cinnamic acid [96]. Methylecgonine (22) undergoes esterification with a benzoyl moiety that was predicted to utilize benzoyl-CoA as the activated acyl donor [97]. It was not determined if it arises from benzoyl-CoA or benzaldehyde, but the moiety was found to be derived from cinnamic acid [96,97]. Acylation reactions of secondary metabolites in plants are catalyzed by several acyltransferase families, however only the BAHD acyltransferase is known to utilize the activated acyl-CoA thioesters [98]. TAs are modified through the esterification of the hydroxyl function at the C3 position in the tropane ring. It has been established that in E. coca, the cocaine synthase reaction uses benzoyl-CoA and methylecgonine (22) as substrates. With the CoA-dependent nature of this enzyme along with the reported properties for the tigloyl-CoA:pseudotropine acyltransferase from D. stramonium, it was hypothesized that cocaine synthase is a member of the BAHD acyltransferase superfamily [99]. This superfamily of enzymes is well known to participate in secondary metabolite modification of esters and amides [98]. Cocaine synthase was found to be capable of producing both cocaine (1) via activated benzoyl-CoA thioester and cinnamoylcocaine via activated cinnamoyl-CoA thioesters [100]. It has been determined that the acylation of the 3β-hydroxyl function of methylecgonine (22), catalyzed by cocaine synthase, forms cocaine (1) and other TAs in E. coca. The accumulation and biosynthesis of TAs in E. coca occur within the same tissue. Cocaine synthase is found localized in the parenchyma and spongy mesophyll of the leaves. These tissues are both responsible for the biosynthesis and storage of TAs in this species. 4-coumaroylquinate has been reported to assist in the storage of cocaine (1) and cinnamoylcocaine in E. coca [101]. This is in contrast to TAs produced in the Solanaceae family where the core biosynthetic pathway is in the roots, while the metabolites accumulate in the aboveground portions of the plants [70].
The rearrangement of the hydroxyl group of the phenyllactic acid moiety of littorine (24) in TA side chain biosynthesis has drawn interest. The phenomenon occurs in both atropine and scopolamine (3) biosynthesis. A branched-chain residue, tropic acid, is formed from the linear chain phenyllactic acid. To better understand this process, feeding studies of radiolabeled compounds have tried to elucidate the mechanism of this reaction [102,103,104,105,106]. Feeding studies and quantum chemistry calculations by Sandala et al. have led to the hypothesis that a cytochrome p450 coupled with an alcohol dehydrogenase is involved in the conversion of the littorine (24) precursor into hyoscyamine (4) [107]. Li et al. were able to suppress cytochrome p450 CYP80F1 expression by using virus-induced gene silencing techniques that caused reduced levels of hyoscyamine (4) and promoted the accumulation of littorine (24) [108]. Using arylfluorinated analogues of (R)- and (S)-littorine, Nasomajai et al. were able to determine that the CYP80F1 catalyzed hydroxylation occurs via a benzylic carbocation intermediate [109]. Reversible 3′-acetoxylation of hyoscyamine (4) is thought to control the flux from hyoscyamine (4) to scopolamine (3) [110].
Hyoscyamine (4) is converted into the epoxide scopolamine (3) via hyoscyamine 6β-hydroxylase (H6H). This enzyme was shown to be a 2-oxoglutarate-dependent dioxygenase from purified H. niger [111,112]. Hydroxylation at the C6 position of hyoscyamine (4) followed by the epoxidation of anisodamine (2) (6β-hydroxy hyoscyamus) is catalyzed by H6H. The localization of this enzyme was also determined to be exclusively in the pericycle of roots [113]. Some solanaceous species contain acylations at the C6, C7 and C3 positions [110]. In a recent networking analysis study of the tropane biosynthetic pathway in D. innoxia, an enzyme activity was theorized in which acylation at the C3 position with a tiglic acid occurs using a C6 acylated tropane as the substrate [110]. By extension, Nguyen et al. have suggested that there are alternate C6-hydroxylating enzymes present with specificities different from H6H that could use tropinone (23) as a substrate instead of the reduced tropine (13) derivative. The same networking study revealed that high variability exists for the acylated tropanes which directly contributes to their chemical diversity [110]. Interestingly, some of the 3-hydroxyl acylating enzymes appear to not be stereospecific. Such enzymes could be useful for expanding the biocatalytic tools available to those interested in metabolic engineering or synthetic biology of tropane derivatives.
3. Granatane Alkaloid Biosynthesis
In this review, our definition of GAs includes piperideine derived compounds that incorporate a pelletierine (5) or N-methylpelletierine (7) core structure. There has been some confusion among scientists regarding the naming and configuration of pelletierine (5). Older reports sometimes use the name isopelletierine incorrectly to refer to the compound [R-1-(2-piperidyl) propan-2-one], which in reality corresponds to pelletierine (5). The name isopelletierine is the optically inactive racemate of pelletierine (5) [114]. The correct chemical name for the compound N-methylpelletierine (7) is 1-[(2R)-1-methyl-2piperdinyl]-2-propanone. Pseudopelletierine (6), also referred to as granatanone, contains a bicyclic core and can be referred to as [9-methyl-9-azabicyclo [1,3,3] nonan-3-one]. Additionally, the presence of anabasine (8), in Nicotiana species would by extension mean that selected members of the Solanaceae can be classified as granatane producing members. Furthermore, the solanaceous species W. somifera produces anabasine (8), which could also be classified as a GA. In addition, several Sedum species (family Crassulaceae) produce the compounds pelletierine (5), N-methylpelletierine (7), and pseudopelletierine (6). All of the species discussed above are members of the order Solanales and are more closely related to one another than they are to other granatane producing angiosperms (Figure 3). This may suggest that, at least within this order of angiosperms, the biosynthetic pathways leading to either tropanes or granatanes are commonly derived. The last time members of the Solanales shared a common ancestor with granatane producing lines in the Myrtales is approximately 120 million years ago. This is similar to the distance between the tropane producing Erythroxylaceae and Solanaceae.
The only research studies performed with the aim to demonstrate the biochemical precursors of GAs have been performed by feeding plants radiolabeled precursors. Table 1 provides a comprehensive summary of feeding studies performed in granatane-producing species as of this review. The labeled products were then subjected to analysis via chemical breakdown and modification. While these studies were informative, they can also be misleading when attempting to interpret them through the viewpoint of biochemical enzymes and mechanisms. The general consensus is that lysine (25) is the starting substrate for the entry into granatane biosynthesis. In several cases, different forms of labeled lysine were found incorporated into the core granatane structure [115,116,117,118,119,120].

Table 1.
Summary of radiolabel feeding studies performed on granatane-producing species.
Over the course of studies regarding granatane and piperideine alkaloid biosynthesis, there has been some controversy over the origin of the intermediates in the biosynthetic pathway. One of the main questions that has repeatedly been tested is whether or not cadaverine (26) is present in the pathway to form pelletierine (5) and other granatane derivatives. Several contradictory observations have been made depending on the type of label of the compound fed to plants as well as which species was used. The majority of the debate regarding the beginning intermediates arises when older radiolabel feeding studies in the Sedum species are used as a reference. These studies report the asymmetrical incorporation of the starting precursors into their respective alkaloids. However, other studies performed on pomegranate report that a symmetrical intermediate (such as cadaverine (26)) must exist [122]. Possible explanations for this problem could be that the members of the family Crassulaceae (e.g., Sedum species) do not utilize the same enzymatic steps as other granatane producing members such as those found in the family Lythraceae.
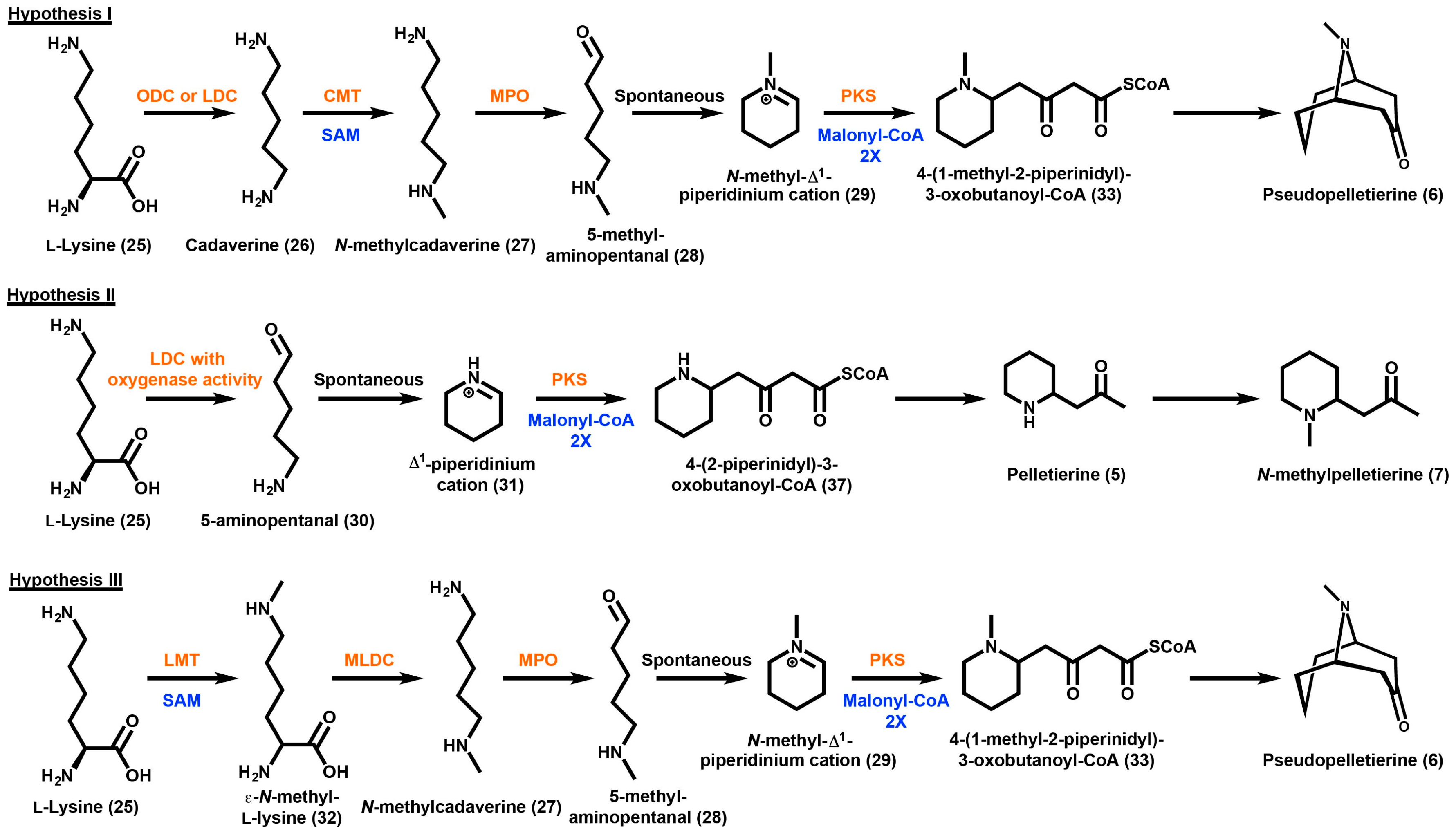
The three hypothesized biosynthetic pathways for the production of GAs will be referred to as Hypothesis I, Hypothesis II, and Hypothesis III; their schematic representation is shown in Scheme 5. Hypothesis I is based on the observation that P. granatum plants fed [1.5-14C] and [1.5-14C 3H2] cadaverine yields incorporation of the labels into N-methylpelletierine (7) and pseudopelletierine (6) [121]. In this pathway, lysine (25) is first decarboxylated to form cadaverine (26) which would subsequently be methylated to form N-methylcadaverine (27) [119]. N-methylcadaverine (27) would then be oxidized to form 5-methylaminopentanal (28) promoting a spontaneous cyclization to form the N-methyl-∆1-piperidinium cation (29). This product would then ultimately form N-methylpelletierine (7) or be converted to the corresponding oxobutanoate by the decarboxylative condensation of two malonyl-CoA which would then cyclize to form pseudopelletierine (6). Feeding studies performed in Sedum species report that cadaverine (26) is not present as an intermediate, giving rise to Hypothesis II. By feeding [1-14C] cadaverine, Leistner et al. (1990) have predicted that there should be a lysine decarboxylase enzyme that also contains oxidase activity such that the only cadaverine (26) intermediate that exists is always enzyme bound [122]. Therefore, according to this hypothesis, lysine (25) is catalyzed in one enzyme or enzyme complex to produce 5-aminopentanal (30). The 5-aminopentanal intermediate (30) would cyclize to form a ∆1-piperidinium cation (31), which can then be methylated at the nitrogen atom to form the N-methyl-∆1-piperidinium cation (29) or remain unmethylated and ultimately go on to form pelletierine (5).
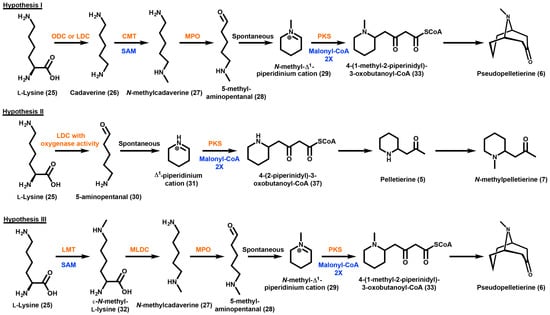
Scheme 5.
Three proposed hypothetical routes to the production of granatane alkaloids. Hypothesized enzymes are presented in orange and hypothetical cofactors are presented in blue. The abbreviated enzymes and cofactor are as follows: ODC, ornithine decarboxylase; LDC, lysine decarboxylase; CMT, cadaverine N-methyl transferase; MPO, methyl putrescine oxidase; PKS, polyketide synthase; LMT, lysine N-methyl transferase; MLDC, N-methyllysine decarboxylase; SAM, S-adenosyl-l-methionine.
In all three hypotheses the origin of the N-methyl group is via labeled methionine [121]. The biochemical methyl donor for this incorporation would be S-adenosylmethionine (SAM). Hypothesis III also involves an early asymmetrical intermediate. In this hypothesis, the first biosynthetic step is the methylation of lysine (25) which occurs at the ε-N position producing ε-N-methyl-lysine (32). This compound would then be decarboxylated to form N-methylcadaverine (27) which would undergo oxidation to form 5-methylaminopentanal (28), promoting a spontaneous cyclization to form the N-methyl-∆1-piperidinium cation (29). As described in Hypothesis I, the N-methyl-∆1-piperidinium cation (29) would ultimately form N-methylpelletierine (7) and pseudopelletierine (6).
Radioactive acetate is incorporated into GAs regardless of which species is fed [116,117,118,119]. As is the case with tropane alkaloid biosynthesis, the enzymes involved in the extension and cyclization reactions of granatanes includes a putative polyketide synthase. Plants readily convert acetate into malonyl-CoA via the enzyme acetyl-CoA carboxylase [123]. A type III polyketide synthase utilizing 2 units of malonyl-CoA and the N-methyl-∆1-piperidinium cation (29) would produce 4-(1-methyl-2-piperinidyl)-3-oxobutanoyl-CoA (33). The prediction of the presence of a type III polyketide synthase in GA biosynthesis is supported by radiolabeling studies which fed ethyl (R,S)-[2,3-13C2,3-14C-]-4-(l-methyl-2-pyrrolidinyl)-3-oxobutanoate to D. stramonium plants [77,80,83]. The oxobutanoyl compound may then have several fates; its conversion to pseudopelletierine (6) by intermolecular interaction of the positively charged nitrogen and the carboxyl CoA, or its conversion to N-methylpelletierine (7) or pelletierine (5) by losing the carboxyl as CO2. Bicyclic GA producing species are similar to solanaceous plants producing tropanes, namely the loss of the carboxyl group due to a lack of methyl ester protection. As mentioned earlier in this review, the protection of the carboxyl group of oxobutanoate by methylation gives rise to the moiety responsible for the narcotic effects of cocaine (1), which would explain why GAs do not exhibit narcotic effects [18].
The enzymes responsible for carrying out the biochemical reactions described above are based on an extension of similar reactions carried out in tropane producing species. The decarboxylation of lysine (25) described in Hypothesis I would be performed by a P. granatum lysine decarboxylase (LDC). The presence of a lysine decarboxylase in the synthesis of anabasine (8) in Nicotiana species has been studied by the incorporation of [15N]-lysine into anabasine (8) [124]. The methylation of cadaverine (26) would be achieved with the help of a P. granatum cadaverine N-methyltransferase (CMT). CMTs have not been isolated and characterized in other species, but this enzyme is predicted to be related to putrescine N-methyltransferases (PMT) and spermidine synthases (SPDS). PMT cDNA sequences have been found in Nicotiana species by the sequencing of large genomic libraries [125,126]. The enzymes SPDS and PMT have been found to share substrate specificity, but PMT is dependent of the decarboxylated S-adenosylmethionine (dcSAM) as a co-substrate. The oxidation of N-methylcadaverine (27) in P. granatum and Sedum species could be performed with the aid of an enzyme similar to the methylputrescine oxygenase (MPO) present in the biosynthesis of nicotine in N. tabacum. While a copper dependent oxidase is used for tropane alkaloid biosynthesis, it is not possible to rule out alternative enzymes such as polyamine oxidases that require FAD as a cofactor [127]. The methylation of lysine (25) described in Hypothesis III would be performed by a lysine methyltransferase (LMT). The responsible enzyme may be related to the lysine methyltransferases ubiquitously present in eukaryotic primary metabolism for gene access regulation to chromatin [128].
In both granatane and tropane alkaloid producing species, dimerized versions of intermediates within their respective pathways have been found. For example, cuscohygrine is the dimerized form of hygrine (20) [129]. Originally it was believed that hygrine (20) was a true intermediate of the biosynthesis of TAs. However, it is most likely that cuscohygrine is a dimerized product of hygrine (20) which in turn is a breakdown product of 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoyl-CoA (34). If this compound is present as a free acid under physiological conditions a β-ketoester is formed, β-ketoesters very often spontaneously decarboxylate [130]. In the case of GA biosynthesis, anaferine (9) is the dimerization product of pelletierine (5). This also supports the presence of an oxobutanoate intermediate.
4. Metabolic Engineering
There has been an increasing interest in the biosynthesis of tropane alkaloids (TAs), especially to up-regulate the production of valued compounds, such as atropine and scopolamine (3). The World Health Organization (WHO) includes these important pharmaceutical compounds on their list of essential drugs [131]. In normal plant biosynthesis, the yields of the final compounds are in low quantities. Synthesizing TAs chemically in the lab has also been difficult and costly because of their stereochemical nature. Nocquet et al. attempted a total synthesis approach to produce the compound scopolamine (3), however their low yield of 16% does not make this method economically feasible [2]. A major problem for the commercial production of scopolamine (3) in hairy root cultures is achieving industrial level yields [132]. Researchers are now focused on metabolic engineering plants that produce these important compounds to increase final yields, or engineering microorganisms that will be able to produce the compounds from simple sugars or common precursors. A comprehensive table of the most recent metabolic engineering studies targeting specific genes can be seen on Table 2.

Table 2.
A summary of recent metabolic engineering studies targeting specific genes.
Due to its high demand in medicine, scopolamine (3) is the most popular choice for increasing yields via metabolic engineering. Past methods such as genetic breeding, polyploid breeding and radiation breeding have failed to yield a higher content of scopolamine (3) in A. belladonna [138]. Researchers are now focused on the genes encoding rate-limiting enzymes in TA biosynthesis that can be genetically modified in planta. A common focal point centers on what is considered the first and last rate-limiting enzymes in the TA pathway, putrescine N-methyltransferase (PMT) and hyoscyamine 6β-hydroxylase (H6H). The overexpression of only one PMT gene in transgenic hairy root cultures of A. belladonna did not change the total TA content [139]. If the same PMT gene in D. metel was overexpressed, the TA content was significantly increased by almost four times that of the control [140]. H6H has a high catalytic efficiency for converting hyoscyamine (4) to scopolamine (3) [141]. The overexpression of the H6H gene resulted in an increase in the biosynthesis of scopolamine (3) in the transformed TA producing plant, A. belladonna. Another successful use of H6H was in transgenic Hyoscyamus muticus hairy root cultures, where scopolamine (3) levels increased to over 100 times that of the controls [142]. Furthermore, overexpression of H6H in transgenic A. belladonna plants resulted in the leaf and stem alkaloid contents to be exclusively scopolamine (3) [141].
Metabolic engineering endeavors are becoming more complex and are moving away from only modifying one gene at a time. When both PMT and H6H were overexpressed simultaneously in transgenic H. niger root cultures, scopolamine (3) biosynthesis increased to levels over nine times more than the wild type [143]. In an important experiment testing whether metabolic engineering can occur between genes isolated from different species, overexpression of NtPMT and HnH6H in A. belladonna significantly increased scopolamine (3) content of secondary roots when compared to wild-type plants [11]. More studies are needed to understand flux through the tropane biosynthetic pathway in order to increase the overproduction of alkaloids.
D. metel produces important medicinal tropanes and is used by researchers because of its tractable hairy root culture system. Agrobacterium rhizogenes can transform plant roots to hairy roots by utilizing the Ri T-DNA plasmid it carries. Hairy roots that have been induced by A. rhizogenes have high growth rates, are genetically stable, and produce copious amounts of lateral roots [144]. Increased TA biosynthesis correlated with an increase in root biomass. Biotic elicitors, such as yeast extract, bacteria, fungi and viruses, as well as abiotic elicitors, such as metal ions or inorganic components, are used and studied to increase the productivity of hairy roots. These elicitors can trigger different defense responses and phytoalexins in plants as well as improve the release of metabolites into the medium [10]. Shakeran et al. focused on using Staphylococcus aureus and Bacillus cereus as biotic elicitors and silver nitrate and nanosilver as abiotic elicitors on the hairy root cultures of D. metel to see their effects on biomass and atropine production. When live bacteria are present in transformed root cultures, there is a considerable influence on secondary metabolite accumulation [145]. Contrary to this, atropine content in the hairy roots of D. metel infected by B. cereus and S. aureus was reduced more than half when compared to the control. The authors hypothesize that this may be a cause of atropine secretion into the culture medium that was then converted into scopolamine (3) [10]. However, scopolamine (3) was not analyzed in the spent media. The living bacteria can cause various influences on roots, affecting enzymes in the TA pathway to produce alkaloids in D. metel roots [145]. Although atropine accumulation decreased with these biotic elicitors, the biomass of the roots slightly increased approximately 15% when compared to the control [10].
Other attempts to engineer higher TA contents in plants include those that use abiotic elicitors. A summary of recent metabolic engineering studies using elicitors can be seen on Table 3. Silver nitrate can increase anisodamine (2) content in Anisodus acutangulus hairy root cultures [146]. In addition, calcium and nitrate can increase hyoscyamine (4) content in D. stramonium hairy root cultures [147]. Silver nitrate can inhibit the activation of ethylene and in doing so, promotes polyamine synthesis [148,149]. As a consequence, the overall effect of silver nitrate treatment can be seen in the increase of root biomass [148,149,150]. Furthermore, silver nitrate has been demonstrated to elicit the production of phytoalexins which in turn increases TA levels in the root [151].

Table 3.
A summary of recent metabolic engineering studies using elicitors.
Shakeran et al. (2015) used silver nitrate as an abiotic elicitor in D. metel and observed an increase in the transformed hairy root biomass of approximately 16%. However, only half of the expected atropine accumulation was observed in these treatments. Although secretion of atropine was not measured in this study, subsequent reports corroborated this hypothesis by finding a three-fold increase in alkaloids in the spent culture media following silver nitrate treatment [152]. Still, further attempts at increasing alkaloid levels include the treatment using nanosilver particles. These differ from silver nitrate in their physicochemical properties [153,154]. Nanosilver particles adhere strongly to plant tissues and cause an increase in the activation of enzymes involved in secondary metabolite production. This treatment was used successfully in Artemisia annua and D. metel hairy roots increasing tropanes by at least 2.4 fold [10]. Initial polyamine substrates such as putrescine (14) must be present in abundance during TA production for a high yield of the final alkaloid. Currently there are only a few studies attempting to engineer the TA pathway in microorganisms. Qian et al. engineered a strain of Escherichia coli capable of efficiently producing putrescine (14) [134]. However, it was first necessary to reduce the flux of polyamine precursors through a competing pathway. Metabolic pathways for putrescine (14) degradation, uptake and utilization were also deleted. Stress to cells by the overproduction of putrescine (14) was handled by the deletion of RpoS, a stress responsive RNA polymerase sigma factor. To increase the conversion of ornithine (10) to putrescine (14), overexpression of ornithine biosynthetic enzymes and ornithine decarboxylase (ODC) was also necessary. The final metabolically engineered E. coli strain produced 1.68 g/L of putrescine (14) and high cell density cultures (HCDCs) produced 24.2 g/L of putrescine (14). This would be the first step for engineering alkaloid biosynthesis that relies on putrescine (14) in microorganisms. In a follow-up study, Qian et al. (2011) performed similar manipulations to produce a strain of E. coli capable of producing the polyamine cadaverine (26). Introduction of an l-lysine decarboxylase in addition to overexpressing dapA, the gene encoding the enzyme dihydrodipicolinate synthase successfully resulted in the new strain producing as much as 9.61 g/L cadaverine (26) from renewable resources [155]. If metabolic engineers in the future wish to engineer these pathways in other organisms, such as bacteria or yeast, it will be necessary to up-regulate the beginning precursor pathways such that pools of these primary metabolites do not get depleted. Depletion of these essential metabolites would result in the death of the organism during the biosynthesis of these compounds.
Using plant in vitro cell culture or tissue culture is an important tool when studying the regulation and biosynthesis of secondary metabolites. Large quantities of plant material can be produced under controlled and sterile conditions. In tissue cultures of solanaceous plant species, using elicitors mimicking stress hormones can increase important secondary metabolite production [158]. Recently, cell cultures of E. coca in the Erythroxylaceae were used to study TA biosynthesis [157]. Various culture media were tested on their ability to support callus formation as well as cocaine (1) production. The jasmonic acid-isoleucine (JA-Ile) analogue coronalon and salicylic acid (SA) were also used as elicitors to observed their effects on calli metabolism. All three culture media growing calli accumulated cocaine (1). The medium used to grow calli also significantly affected natural product metabolism. The only treatments that yielded higher amounts of cocaine (1) were dependent upon culture media, not upon elicitor treatment. For example, Anderson rhododendron medium (ARM) produced cocaine (1) an order of a magnitude greater than both Gamborg B5 (GB5) and modified Murashige-Tucker medium (MMT), but lower levels of hydroxycinnamate-quinate esters such as chlorogenic acid (CGA) were detected. Interestingly the elicitors coronalon and salicylic acid did not yield any increase in TA production suggesting that TAs, at least in E. coca, may not be regulated by common plant defense hormones.
5. Conclusions
Recent advances in genomics, transcriptomic and metabolomic technologies are poised to illuminate the biosynthetic foundations of TAs and GAs. Future research on the biosynthesis of TAs and GAs will affect multiple fields of research. First, enzymes involved in TA and GA biosynthesis will expand our fundamental knowledge of chemistry and enzymology. Second, elucidation of the genes and enzymes underlying TA and GA biosynthesis will expand the molecular tools available to synthetic biologists. With these additional tools, scientists can look to not only produce TAs and GAs in heterologous hosts but also to engineer novel molecules [159]. Lastly, future discoveries of the function and structure of genes and enzymes in TA and GA biosynthesis will strengthen our understanding of the evolution of plant metabolism. Plant metabolism, especially specialized metabolites such as TAs and GAs, evolved in response to dynamic environments. Detailed knowledge of the catalytic properties of TA and GA biosynthetic enzymes as well as their biophysical properties are critical to our knowledge of the evolution of biochemical activities and the chemical diversity of these metabolic pathways. This fundamental knowledge will be useful in predicting and engineering plants to withstand ongoing changes in the environment.
Acknowledgments
This research was supported by faculty startup funds to JCD from Texas Tech University. BC received support through the Center for Active Learning and Undergraduate Engagement at Texas Tech University.
Author Contributions
All authors contributed to the writing and editing of the text. Neill Kim, Olga Estrada, and Benjamin Chavez contributed to the making and editing of the figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pichersky, E.; Lewinsohn, E. Convergent evolution in plant specialized metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Nocquet, P.-A.; Opatz, T. Total synthesis of (±)-scopolamine: Challenges of the tropane ring. Eur. J. Org. Chem. 2016, 2016, 1156–1164. [Google Scholar] [CrossRef]
- Dillehay, T.D.; Rossen, J.; Ugent, D.; Karathanasis, A.; Vásquez, V.; Netherly, P.J. Early Holocene coca chewing in northern Peru. Antiquity 2010, 84, 939–953. [Google Scholar] [CrossRef]
- Oberlies, N.H.; Paine, M.F. Antimicrobial activities of Pomegranate. In Pomegranates: Ancient Roots to Modern Medicine; Seeram, N.P., Schulman, R.N., Heber, D., Eds.; American Chemical Society: Boca Raton, FL, USA, 2007. [Google Scholar]
- Jirschitzka, J.; Schmidt, G.W.; Reichelt, M.; Schneider, B.; Gershenzon, J.; D’Auria, J.C. Plant tropane alkaloid biosynthesis evolved independently in the Solanaceae and Erythroxylaceae. Proc. Natl. Acad. Sci. USA 2012, 109, 10304–10309. [Google Scholar] [CrossRef] [PubMed]
- Lounasmaa, M.; Tamminen, T. The tropane alkaloids. In The Alkaloids; Cordell, G.A., Ed.; Academic Press: New York, NY, USA, 1993. [Google Scholar]
- Lazny, R.; Ratkiewicz, A.; Nodzewska, A.; Wynimko, A.; Siergiejczyk, L. Determination of the N-methyl stereochemistry in tropane and granatane derivatives in solution: A computational and NMR spectroscopic study. Tetrahedron 2012, 68, 6158–6163. [Google Scholar] [CrossRef]
- Wink, M. Modes of actions of alkaloids. In Alkaloids; Roberts, M.F., Wink, M., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 301–326. [Google Scholar]
- Schmeller, T.; Sporer, F.; Sauerwein, M.; Wink, M. Binding of tropane alkaloids to nicotinic and muscarinic acetylcholine receptors. Pharmazie 1995, 50, 493–495. [Google Scholar] [PubMed]
- Shakeran, Z.; Keyhanfar, M.; Asghari, G.; Ghanadian, M. Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel. Turk. J. Biol. 2015, 39, 111–118. [Google Scholar] [CrossRef]
- Xia, K.; Lui, X.; Zhang, Q.; Qiang, W.; Guo, J.; Lan, X.; Chen, M.; Liao, Z. Promoting scopolamine biosynthesis in transgenic Atropa belladonna plants with pmt and h6h overexpression under field conditions. Plant Physiol. Biochem. 2016, 106, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Xia, K.; Zhang, Q.; Zeng, J.; Huang, Y.; Yang, C.; Chen, M.; Liu, X.; Lan, X.; Liao, Z. Functional characterisation of a tropine-forming reductase gene from Brugmansia arborea, a woody plant species producing tropane alkaloids. Phytochemistry 2016, 127, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lui, W.; Guo, Z.-X.; Chen, B.-L. Fingerprint analysis of Daturae Flos using rapid resolution liquid chromatography-electrospray ionization mass spectrometry combined with stoichiometry. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 137–142. [Google Scholar] [CrossRef]
- Zeng, S.M.; She, Y.X.; Jiao, B.N.; Liu, G.Y.; Wang, J.; Su, X.S.; Ma, X.B.; Jin, M.J.; Jin, F.; Wang, S.S. Molecularly imprinted polymer for selective extraction and simultaneous determination of four tropane alkaloids from Przewalskia tangutica Maxim. fruit extracts using LC-MS/MS. RSC Adv. 2015, 5, 94997–95006. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Liu, G.; Sun, X.; Zhou, Y.; Deng, X.; Liao, Q.; Xie, Z. Simultaneous determination of atropine, scopolamine, and anisodamine from Hyoscyamus niger L. in rat plasma by high-performance liquid chromatography with tandem mass spectrometry and its application to a pharmacokinetics study. J. Sep. Sci. 2014, 37, 2664–2674. [Google Scholar] [CrossRef] [PubMed]
- Mann, J. Murder, Magic, and Medicine; Oxford University Press: New York, NY, USA, 1992; p. 232. [Google Scholar]
- Wink, M. A short history of alkaloids. In Alkaloids; Roberts, M.F., Wink, M., Eds.; Plenum Press: New York, NY, USA, 1998; pp. 11–44. [Google Scholar]
- Carroll, F.I.; Gao, Y.; Abraham, P.; Lewin, A.H.; Lew, R.; Patel, A.; Boja, J.W.; Kuhar, M.J. Probes for the cocaine receptor. Potentially irreversible ligands for the dopamine transporter. J. Med. Chem. 1992, 35, 1813–1817. [Google Scholar] [CrossRef] [PubMed]
- Carroll, F.I.; Lewin, A.H.; Boja, J.W.; Kuhar, M.J. Cocaine receptor: Biochemical characterization and structure-activity relationships of cocaine analogs at the dopamine transporter. J. Med. Chem. 1992, 35, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Sidorowicz, K.; Lazny, R. Structural studies of cyclic β-amino ketons using computational and NMR methods. CHEMIK 2015, 69, 401–410. [Google Scholar]
- Krunic, A.; Pan, D.; Dunn, W.J., 3rd; Mariappan, S.V. The stereochemistry of N-methyl and aryl substituents determine the biological activities of 3-aryl-8-methyl-8-azabicyclo[3.2.1]oct-2,3-enes. Bioorg. Med. Chem. 2009, 17, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Fozard, J. The peripheral actions of 5-hydroxytryptamine. In The Developement and Early Clinical Evaluation of Selective 5-HT3 Receptor Antagonsts; Oxford University Press: Oxford, UK; New York, NY, USA, 1989; pp. 354–376. [Google Scholar]
- Aapro, M. Granisetron: An update on its clinical use in the management of nausea and vomiting. Oncologist 2004, 9, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Balfour, J.A.; Goa, K.L. Dolasetron. A review of its pharmacology and therapeutic potential in the management of nausea and vomiting induced by chemotherapy, radiotherapy or surgery. Drugs 1997, 54, 273–298. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Pae, H.O.; Yoo, J.C.; Kim, N.Y.; Kim, Y.C.; Ko, G.I.; Chung, H.T. Antiproliferative effects of alkaloids from Sedum sarmentosum on murine and human hepatoma cell lines. J. Ethnopharmacol. 2000, 70, 177–182. [Google Scholar] [CrossRef]
- Van Noordwijk, J.; Hollstein, U. The anthelminthic activity of pelletierine and isopelletierine. Acta Physiol. Pharmacol. Neerl. 1956, 5, 212–213. [Google Scholar] [PubMed]
- Van Noordwijk, J.; Mellink, J.J.; Visser, B.J.; Wisse, J.H. Synthesis and anthelmintic activity of isopelletierine and a series of side-chain homologues. Recl. Trav. Chim. Pays-Bas 2010, 82, 763–772. [Google Scholar] [CrossRef]
- Tripathi, S.M.; Singh, D.K. Molluscicidal activity of Punica granatum bark and Canna indica root. Braz. J. Med. Biol. Res. 2000, 33, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.M.; Singh, V.K.; Singh, S.; Singh, D.K. Enzyme inhibition by the molluscicidal agent Punica granatum Linn. Bark and Canna indica Linn. root. Phytother. Res. 2004, 18, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Chidiebere, M.A.; Ogukwe, C.E.; Oguzie, K.L.; Eneh, C.N.; Oguzie, E.E. Corrosion inhibition and adsorption behavior of Punica granatum extract on mild steel in acidic environments: Experimental and theoretical studies. Ind. Eng. Chem. Res. 2012, 51, 668–677. [Google Scholar] [CrossRef]
- Plowman, T. Botanical perspectives on coca. J. Psychedelic Drugs 1979, 11, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Plowman, T.; Hensold, N. Names, types, and distribution of neotropical species of Erythroxylum (Erythroxylaceae). Brittonia 2004, 56, 1–53. [Google Scholar] [CrossRef]
- Plowman, T. The ethnobotany of coca (Erythroxylum spp., Erythroxylaceae). In Ethnobotany in the Neotropics; Prance, G.T., Kallunki, J.A., Eds.; New York Botanical Garden: New York, NY, USA, 1984; pp. 62–111. [Google Scholar]
- Plowman, T. Amazonian coca. J. Ethnopharmacol. 1981, 3, 195–225. [Google Scholar] [CrossRef]
- Niemann, A. Ueber eine neue organische base in den cocablättern. Arch. Pharm. (Weinheim) 1860, 153, 291–308. [Google Scholar] [CrossRef]
- Freud, S. Ueber coca. ZentrBl. Ther. 1884, 2, 289–314. [Google Scholar]
- Plowman, T.; Rivier, L. Cocaine and cinnamoylcocaine content of Erythroxylum species. Ann. Bot. 1983, 51, 641–659. [Google Scholar]
- Naranjo, P. Social function of coca in pre-Columbian America. J. Ethnopharmacol. 1981, 3, 161–172. [Google Scholar] [CrossRef]
- Schmidt, E.; Henschke, H. Über die alkaloide der wurzel von Scopolia japonica. Arch. Pharm. (Weinheim) 1888, 226, 185–203. [Google Scholar] [CrossRef]
- Bisset, N.G. Arrow and dart poisons. J. Ethnopharmacol. 1989, 25, 1–41. [Google Scholar] [CrossRef]
- Schultes, R.E. Hallucinogenic plants. In A Golden Guide; Golden Press: New York, NY, USA, 1976. [Google Scholar]
- Hesse, G. Darstellung des atropins. Ann. Pharm. 1833, 5, 43–81. [Google Scholar]
- Mein. Darstellung des atropins in weissen krystallen. Ann. Pharm. 1833, 6, 67–72. [Google Scholar]
- Chilton, J.; Partridge, M.W. The partition chromatography of alkaloids. Part III—The alkaloids of Punica granatum. J. Pharm. Pharmacol. 1950, 2, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Hess, G.; Haiss, P.; Wistuba, D.; Siehl, H.-U.; Berger, S.; Sicker, D.; Zeller, K.-P. From the pomegranate tree to cyclooctatetraene: Pseudopelletierine. Chem. Unserer Zeit 2016, 50, 34–43. [Google Scholar]
- Khanna, K.L.; Schwarting, A.E.; Bobbitt, J.M. The occurrence of isopelletierine in Withania somnifera. J. Pharm. Sci. 1962, 51, 1194. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; T’Hart, H.; Stevens, J.F. Alkaloids of some Asian Sedum species. Phytochemistry 1996, 41, 1319–1324. [Google Scholar] [CrossRef]
- Chauhan, R.D.; Kanwar, K. Biotechnological advances in pomegranate (Punica granatum L.). In Vitro Cell. Dev. Biol. Plant 2012, 48, 579–594. [Google Scholar] [CrossRef]
- Brachet, A.; Muñoz, O.; Gupta, M.; Veuthey, J.-L.; Christen, P. Alkaloids of Erythroxylum lucidum stem-bark. Phytochemistry 1997, 46, 1439–1442. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Wink, M.; Botschen, F.; Gosmann, C.; Schäfer, H.; Waterman, P.G. Chemotaxonomy seen from a phylogenetic perspective and evolution of secondary metabolism. In Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism; Wink, M., Ed.; Wiley-Blackwell: Oxford, UK, 2010; Volume 40, pp. 364–433. [Google Scholar]
- Lazny, R.; Sienkiewicz, M.; Olenski, T.; Urbanczyk-Lipkowska, Z.; Kalicki, P. Approaches to the enantioselective synthesis of ferrugine and its analogues. Tetrahedron 2012, 68, 8236–8244. [Google Scholar] [CrossRef]
- Brock, A.; Herzfeld, T.; Paschke, R.; Koch, M.; Drager, B. Brassicaceae contain nortropane alkaloids. Phytochemistry 2006, 67, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Magallon, S.; Castillo, S. Angiosperm diversification through time. Am. J. Bot. 2009, 96, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Leete, E.; Marion, L.; Spenser, I.D. The biogenesis of alkaloids: 12. The mode of formation of the tropine base of hyoscyamine. Can. J. Chem. Rev. Can. Chim. 1954, 32, 1116–1123. [Google Scholar] [CrossRef]
- Liebisch, H.W.; Schütte, H.R. Zur biosynthese der tropanalkaloide. VIII. Vorstufen des pyrrolidinringes. Z. Pflanzenphysiol. 1967, 57, 434–439. [Google Scholar]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Leete, E. Recent developments in the biosynthesis of the tropane alkaloids. Planta Med. 1990, 56, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Leete, E. Stereospecific incorporation of ornithine into tropine moiety of hyoscyamine. J. Am. Chem. Soc. 1962, 84, 55–57. [Google Scholar] [CrossRef]
- Leete, E. Biosynthesis of the pyrrolidine rings of cocaine and cuscohygrine from [5-14C]-labeled ornithine via a symmetrical intermediate. J. Am. Chem. Soc. 1982, 104, 1403–1408. [Google Scholar] [CrossRef]
- Docimo, T.; Reichelt, M.; Schneider, B.; Kai, M.; Kunert, G.; Gershenzon, J.; D’Auria, J.C. The first step in the biosynthesis of cocaine in Erythroxylum coca: The characterization of arginine and ornithine decarboxylases. Plant Mol. Biol. 2012, 78, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, R.L.; Watson, M.B.; Galloway, G.L.; Yu, W. Molecular genetic analyses of plant polyamines. CRC Crit. Rev. Plant Sci. 1998, 17, 199–224. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, B.; Lu, B.; Kai, G.; Wang, Z.; Xia, Y.; Ding, R.; Zhang, H.; Sun, X.; Chen, W.; et al. Tropane alkaloids production in transgenic Hyoscyamus niger hairy root cultures over-expressing putrescine N-methyltransferase is methyl jasmonate-dependent. Planta 2007, 225, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Hibi, N.; Higashiguchi, S.; Hashimoto, T.; Yamada, Y. Gene expression in tobacco low-nicotine mutants. Plant Cell 1994, 6, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Galston, A.W.; Sawhney, R.K. Polyamines in plant physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Tamaki, K.; Suzuki, K.; Yamada, Y. Molecular cloning of plant spermidine synthases. Plant Cell Physiol. 1998, 39, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamada, Y.; Hashimoto, T. Expression of Atropa belladonna putrescine N-methyltransferase gene in root pericycle. Plant Cell Physiol. 1999, 40, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Hashimoto, T. Two tropinone reductases, that catalyze opposite stereospecific reductions in tropane alkaloid biosynthesis, are localized in plant root with different cell-specific patterns. Plant Cell Physiol. 1999, 40, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769. [Google Scholar] [CrossRef] [PubMed]
- Leete, E. Spermidine: An indirect precursor of the pyrrolidine rings of nicotine and nornicotine in Nicotiana glutinosa. Phytochemistry 1985, 24, 957–960. [Google Scholar] [CrossRef]
- Matsuzaki, R.; Fukui, T.; Sato, H.; Ozaki, Y.; Tanizawa, K. Generation of the topa quinone cofactor in bacterial monoamine oxidase by cupric ion-dependent autooxidation of a specific tyrosyl residue. FEBS Lett. 1994, 351, 360–364. [Google Scholar] [CrossRef]
- Heim, W.G.; Sykes, K.A.; Hildreth, S.B.; Sun, J.; Lu, R.H.; Jelesko, J.G. Cloning and characterization of a Nicotiana tabacum methylputrescine oxidase transcript. Phytochemistry 2007, 68, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Katoh, A.; Shoji, T.; Hashimoto, T. Molecular cloning of N-methylputrescine oxidase from tobacco. Plant Cell Physiol. 2007, 48, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Bjorklund, J.A.; Koltun, D.O.; Renner, M.K. N-methylputrescine oxidation during cocaine biosynthesis: Study of prochiral methylene hydrogen discrimination using the remote isotope method. Org. Lett. 2000, 2, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Romek, K.M.; Remaud, G.S.; Silvestre, V.; Paneth, P.; Robins, R.J. Non-statistical 13C fractionation distinguishes co-incident and divergent steps in the biosynthesis of the alkaloids nicotine and tropine. J. Biol. Chem. 2016, 291, 16620–16629. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.W.; Leete, E. New intermediate in the biosynthesis of the tropane alkaloids in Datura innoxia. J. Am. Chem. Soc. 1995, 117, 8100–8105. [Google Scholar] [CrossRef]
- Kaczkowski, J.; Schütte, H.R.; Mothes, K. Die rolle des acetats in der biosynthese der tropanalkaloide. Biochim. Biophys. Acta 1961, 46, 588–594. [Google Scholar] [CrossRef]
- Liebisch, H.W.; Peisker, K.; Radwan, A.S.; Schütte, H.R. Zur biosynthese der tropanalkaloide. XI. Die bildung der C3-brücke des tropins. Z. Pflanzenphysiol. 1972, 67, 1–9. [Google Scholar] [CrossRef]
- Robins, R.J.; Abraham, T.W.; Parr, A.J.; Eagles, J.; Walton, N.J. The biosynthesis of tropane alkaloids in Datura stramonium: The identity of the intermediates between N-methylpyrrolinium salt and tropinone. J. Am. Chem. Soc. 1997, 119, 10929–10934. [Google Scholar] [CrossRef]
- Leete, E.; Bjorklund, J.A.; Couladis, M.M.; Kim, S.H. Late intermediates in the biosynthesis of cocaine: 4-(1-Methyl-2-pyrrolidinyl)-3-oxobutanoate and methyl ecgonine. J. Am. Chem. Soc. 1991, 113, 9286–9292. [Google Scholar] [CrossRef]
- Hagel, J.M.; Facchini, P.J. Benzylisoquinoline alkaloid metabolism: A century of discovery and a brave new world. Plant Cell Physiol. 2013, 54, 647–672. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, A.J.; O’Hagan, D. Tropane alkaloid biosynthesis. A century old problem unresolved. Nat. Prod. Rep. 2001, 18, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C., Jr.; Vickery, C.R.; Burkart, M.D.; Noel, J.P. Confluence of structural and chemical biology: Plant polyketide synthases as biocatalysts for a bio-based future. Curr. Opin. Plant Biol. 2013, 16, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M.; Bowman, M.E.; Noel, J.P. Expanding the biosynthetic repertoire of plant type III polyketide synthases by altering starter molecule specificity. Proc. Natl. Acad. Sci. USA 2002, 99, 5319–5324. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Shimokawa, Y.; Matsui, T.; Kinjo, K.; Kato, R.; Noguchi, H.; Sugio, S.; Morita, H.; Abe, I. Cloning and structure-function analyses of quinolone- and acridone-producing novel type III polyketide synthases from Citrus microcarpa. J. Biol. Chem. 2013, 288, 28845–28858. [Google Scholar] [CrossRef] [PubMed]
- Resmi, M.S.; Verma, P.; Gokhale, R.S.; Soniya, E.V. Identification and characterization of a type III polyketide synthase involved in quinolone alkaloid biosynthesis from Aegle marmelos Correa. J. Biol. Chem. 2013, 288, 7271–7281. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.L.; Jornvall, H.; Persson, B.; Oppermann, U. Medium- and short-chain dehydrogenase/reductase gene and protein families: The SDR superfamily: Functional and structural diversity within a family of metabolic and regulatory enzymes. Cell. Mol. Life Sci. 2008, 65, 3895–3906. [Google Scholar] [CrossRef] [PubMed]
- Dräger, B. Tropinone reductases, enzymes at the branch point of tropane alkaloid metabolism. Phytochemistry 2006, 67, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Moummou, H.; Kallberg, Y.; Tonfack, L.B.; Persson, B.; van der Rest, B. The plant short-chain dehydrogenase (SDR) superfamily: Genome-wide inventory and diversification patterns. BMC Plant Biol. 2012, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kato, H.; Oda, J.; Yamada, Y.; Hashimoto, T. Site-directed mutagenesis of putative substrate-binding residues reveals a mechanism controlling the different stereospecificities of two tropinone reductases. J. Biol. Chem. 1999, 274, 16563–16568. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Hashimoto, T.; Yamada, Y. Two tropinone reductases with different stereospecificities are short-chain dehydrogenases evolved from a common ancestor. Proc. Natl. Acad. Sci. USA 1993, 90, 9591–9595. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M.; Bennett, M.J.; Schlegel, B.P.; Lewis, M.; Penning, T.M. Comparative anatomy of the aldo-keto reductase superfamily. Biochem. J. 1997, 326, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.; Clouet, D.H.; Misra, A.L.; Mule, S. Cocaine and metabolites—Relationship between pharmacological activity and inhibitory action on dopamine uptake into striatal synaptosomes. Prog. Neuropsychopharmacol. 1977, 1, 265–269. [Google Scholar] [CrossRef]
- Bjorklund, J.A.; Leete, E. Biosynthesis of the benzoyl moiety of cocaine from cinnamic acid via (R)-(+)-3-hydroxy-3-phenylpropanoic acid. Phytochemistry 1992, 31, 3883–3887. [Google Scholar] [CrossRef]
- Leete, E.; Bjorklund, J.A.; Kim, S.H. The biosynthesis of the benzoyl moiety of cocaine. Phytochemistry 1988, 27, 2553–2556. [Google Scholar] [CrossRef]
- D’Auria, J.C. Acyltransferases in plants: A good time to be BAHD. Curr. Opin. Plant Biol. 2006, 9, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Rabot, S.; Peerless, A.C.J.; Robins, R.J. Tigloyl-CoA: Pseudotropine acyl transferase—An enzyme of tropane alkaloid biosynthesis. Phytochemistry 1995, 39, 315–322. [Google Scholar] [CrossRef]
- Schmidt, G.W.; Jirschitzka, J.; Porta, T.; Reichelt, M.; Luck, K.; Pardo Torre, J.C.; Dolke, F.; Varesio, E.; Hopfgartner, G.; Gershenzon, J.; et al. The last step in cocaine biosynthesis is catalyzed by a BAHD acyltransferase. Plant Physiol. 2015, 167, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Torre, J.C.; Schmidt, G.W.; Paetz, C.; Reichelt, M.; Schneider, B.; Gershenzon, J.; D’Auria, J.C. The biosynthesis of hydroxycinnamoyl quinate esters and their role in the storage of cocaine in Erythroxylum coca. Phytochemistry 2013, 91, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Leete, E.; Kowanko, N.; Newmark, R.A. Use of carbon-13 nuclear magnetic-resonance to establish that biosynthesis of tropic acid involves an intramolecular rearrangement of phenylalanine. J. Am. Chem. Soc. 1975, 97, 6826–6830. [Google Scholar] [CrossRef] [PubMed]
- Ansarin, M.; Woolley, J.G. The rearrangement of phenyllactate in the biosynthesis of tropic acid. Phytochemistry 1994, 35, 935–939. [Google Scholar] [CrossRef]
- Robins, R.J.; Bachmann, P.; Woolley, J.G. Biosynthesis of hyoscyamine involves an intramolecular rearrangement of littorine. J. Chem. Soc. Perkin Trans. 1994, 25, 615–619. [Google Scholar] [CrossRef]
- Chesters, N.C.J.E.; O’Hagan, D.; Robins, R.J. The biosynthesis of tropic acid: The (R)-d-phenyllactyl moiety is processed by the mutase involved in hyoscyamine biosynthesis in Datura stramonium. J. Chem. Soc. Chem. Commun. 1995, 2, 127–128. [Google Scholar] [CrossRef]
- Robins, R.J.; Chesters, N.C.J.E.; O’Hagan, D.; Parr, A.J.; Walton, N.J.; Woolley, J.G. The biosynthesis of hyoscyamine: The process by which littorine rearranges to hyoscyamine. J. Chem. Soc. Perkin Trans. 1995, 4, 481–485. [Google Scholar] [CrossRef]
- Sandala, G.M.; Smith, D.M.; Radom, L. The carbon-skeleton rearrangement in tropane alkaloid biosynthesis. J. Am. Chem. Soc. 2008, 130, 10684–10690. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Reed, D.W.; Liu, E.W.; Nowak, J.; Pelcher, L.E.; Page, J.E.; Covello, P.S. Functional genomic analysis of alkaloid biosynthesis in Hyoscyamus niger reveals a cytochrome p450 involved in littorine rearrangement. Chem. Biol. 2006, 13, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Nasomjai, P.; Reed, D.W.; Tozer, D.J.; Peach, M.J.G.; Slawin, A.M.Z.; Covello, P.S.; O’Hagan, D. Mechanistic insights into the cytochrome p450-mediated oxidation and rearrangement of littorine in tropane alkaloid biosynthesis. ChemBioChem 2009, 10, 2382–2393. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Jamali, A.; Lanoue, A.; Gontier, E.; Dauwe, R. Unravelling the architecture and dynamics of tropane alkaloid biosynthesis pathways using metabolite correlation networks. Phytochemistry 2015, 116, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Yamada, Y. Hyoscyamine 6b-hydroxylase, a 2-oxoglutarate-dependent dioxygenase, in alkaloid-producing root cultures. Plant Physiol. 1986, 81, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Matsuda, J.; Yamada, Y. Two-step epoxidation of hyoscyamine to scopolamine is catalyzed by bifunctional hyoscyamine 6b-hydroxylase. FEBS Lett. 1993, 329, 35–39. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hayashi, A.; Amano, Y.; Kohno, J.; Iwanari, H.; Usuda, S.; Yamada, Y. Hyoscyamine 6b-hydroxylase, an enzyme involved in tropane alkaloid biosynthesis, is localized at the pericycle of the root. J. Biol. Chem. 1991, 266, 4648–4653. [Google Scholar] [PubMed]
- Beyerman, H.C.; Maat, L. Resolution of isopelletierine: A second synthesis of pelletierine. Recl. Trav. Chim. Pays-Bas 1965, 84, 385–388. [Google Scholar] [CrossRef]
- Leistner, E.; Gupta, R.N.; Spenser, I.D. A general method for the determination of precursor configuration in biosynthetic precursor-product relationships. Derivation of pipecolic acid from d-lysine, and of piperidine alkaloids from l-lysine. J. Am. Chem. Soc. 1973, 95, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Hemscheidt, T.; Spenser, I.D. Biosynthesis of N-methylpelletierine: Vindication of a classical biogenetic concept. J. Am. Chem. Soc. 1990, 112, 6360–6363. [Google Scholar] [CrossRef]
- Gupta, R.N.; Spenser, I.D. Biosynthesis of N-methylpelletierine. Phytochemistry 1969, 8, 1937–1944. [Google Scholar] [CrossRef]
- Keogh, M.F.; Odonovan, D.G. Biosynthesis of some alkaloids of Punica granatum and Withania somnifera. J. Chem. Soc. C 1970, 13, 1792–1797. [Google Scholar] [CrossRef]
- O’Donovan, D.G.; Keogh, M.F. Biosynthesis of piperidine alkaloids. Tetrahedron Lett. 1968, 3, 265–267. [Google Scholar] [CrossRef]
- Gupta, R.N.; Spenser, I.D. Biosynthesis of the piperidine alkaloids. Origin of the piperidine nucleus of N-methylisopelletierine. Chem. Commun. 1968, 2, 85–86. [Google Scholar] [CrossRef]
- Liebisch, H.W.; Marekov, N.; Schutte, H.R. Biosynthesis of alkaloids from Punica granatum. Z. Naturforsch. B 1968, 23, 1116–1117. [Google Scholar] [CrossRef] [PubMed]
- Leistner, E.; Spenser, I.D. Biosynthesis of the piperidine nucleus. Incorporation of chirally labeled cadaverine-1–3H. J. Am. Chem. Soc. 1973, 95, 4715–4725. [Google Scholar] [CrossRef] [PubMed]
- Shorrosh, B.S.; Dixon, R.A.; Ohlrogge, J.B. Molecular cloning, characterization, and elicitation of acetyl-CoA carboxylase from alfalfa. Proc. Natl. Acad. Sci. USA 1994, 91, 4323–4327. [Google Scholar] [CrossRef] [PubMed]
- Bunsupa, S.; Komastsu, K.; Nakabayashi, R.; Saito, K.; Yamasaki, M. Revisiting anabasine biosynthesis in tobacco hairy roots expressing plant lysine decarboxylase gene by using 15N-labeled lysine. Phytochemistry 2014, 31, 511–588. [Google Scholar]
- Riechers, D.E.; Timko, M.P. Structure and expression of the gene family encoding putrescine N-methyltransferase in Nicotiana tabacum: New clues to the evolutionary origin of cultivated tobacco. Plant Mol. Biol. 1999, 41, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Winz, R.A.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. IV. Insect-induced ethylene reduces jasmonate-induced nicotine accumulation by regulating putrescine N-methyltransferase transcripts. Plant Physiol. 2001, 125, 2189–2202. [Google Scholar] [PubMed]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Pontvianne, F.; Blevins, T.; Pikaard, C.S. Arabidopsis histone lysine methyltransferases. Adv. Bot. Res. 2010, 53, 1–22. [Google Scholar] [PubMed]
- Leete, E. Biosynthesis of cocaine and cuscohygrine in Erythroxylon coca. J. Chem. Soc. Chem. Commun. 1980, 22, 1170–1171. [Google Scholar] [CrossRef]
- Yu, G.; Nguyen, T.T.H.; Guo, Y.; Schauvinhold, I.; Auldridge, M.E.; Bhuiyan, N.; Ben-Israel, I.; Iijima, Y.; Fridman, E.; Noel, J.P.; et al. Enzymatic functions of wild tomato methylketone synthases 1 and 2. Plant Physiol. 2010, 154, 67–77. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Who Model List of Essential Medicines, 17th List (March 2011); World Health Organization: Geneva, Switzerland, 2011; p. 41. [Google Scholar]
- Ullrich, S.F.; Hagels, H.; Kayser, O. Scopolamine: A journey from the field to clinics. Phytochem. Rev. 2016. [Google Scholar] [CrossRef]
- Wang, X.; Chen, M.; Yang, C.; Liu, X.; Zhang, L.; Lan, X.; Tang, K.; Liao, Z. Enhancing the scopolamine production in transgenic plants of Atropa belladonna by overexpressing pmt and h6h genes. Physiol. Plant. 2011, 143, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.G.; Xia, X.X.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of putrescine: A four carbon diamine. Biotechnol. Bioeng. 2009, 104, 651–662. [Google Scholar] [PubMed]
- Cardillo, A.B.; Giulietti, A.M.; Palazon, J.; Bonfill, M. Influence of hairy root ecotypes on production of tropane alkaloids in Brugmansia candida. Plant Cell Tissue Organ Cult. 2013, 114, 305–312. [Google Scholar] [CrossRef]
- Richter, U.; Rothe, G.; Fabian, A.K.; Rahfeld, B.; Drager, B. Overexpression of tropinone reductases alters alkaloid composition in Atropa belladonna root cultures. J. Exp. Bot. 2005, 56, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kai, G.; Yang, S.; Luo, X.; Zhou, W.; Fu, X.; Zhang, A.; Zhang, Y.; Xiao, J. Co-expression of AaPMT and AaTRI effectively enhances the yields of tropane alkaloids in Anisodus acutangulus hairy roots. BMC Biotechnol. 2011, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-H.; Pan, X.-C.; Qiang, W.; Liao, Z.-H. Improvement of biosynthesis of tropane alkaloids in Anisodus acutangulus by Co-transformed PMT and H6H. Plant Omics 2014, 36, 21–27. [Google Scholar]
- Rothe, G.; Hachiya, A.; Yamada, Y.; Hashimoto, T.; Drager, B. Alkaloids in plants and root cultures of Atropa belladonna overexpressing putrescine N-methyltransferase. J. Exp. Bot. 2003, 54, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Moyano, E.; Jouhikainen, K.; Tammela, P.; Palazón, J.; Cusidó, R.M.; Piñol, M.T.; Teeri, T.H.; Oksman-Caldentey, K.-M. Effect of pmt gene overexpression on tropane alkaloid production in transformed root cultures of Datura metel and Hyoscyamus muticus. J. Exp. Bot. 2003, 54, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.J.; Hashimoto, T.; Yamada, Y. Metabolic engineering of medicinal plants: Transgenic Atropa belladonna with an improved alkaloid composition. Proc. Natl. Acad. Sci. USA 1992, 89, 11799–11803. [Google Scholar] [CrossRef] [PubMed]
- Jouhikainen, K.; Lindgren, L.; Jokelainen, T.; Hiltunen, R.; Teeri, T.H.; Oksman-Caldentey, K.-M. Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta 1999, 208, 545–551. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, R.; Chai, Y.; Bonfill, M.; Moyano, E.; Oksman-Caldentey, K.-M.; Xu, T.; Pi, Y.; Wang, Z.; Zhang, H.; et al. Engineering tropane biosynthetic pathway in Hyoscyamus niger hairy root cultures. Proc. Natl. Acad. Sci. USA 2004, 101, 6786–6791. [Google Scholar] [CrossRef] [PubMed]
- Archana Giri, M.L.N. Transgenic hairy roots: Recent trends and applications. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ng, J.; Shi, M.; Wu, S.J. Enhanced secondary metabolite (tanshinone) production of Salvia miltiorrhiza hairy roots in a novel root-bacteria coculture process. Appl. Microbiol. Biotechnol. 2007, 77, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Kai, G.; Yang, S.; Zhang, Y.; Luo, X.; Fu, X.; Zhang, A.; Xiao, J. Effects of different elicitors on yield of tropane alkaloids in hairy roots of Anisodus acutangulus. Mol. Biol. Rep. 2012, 39, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Amdoun, R.; Khelifi, L.; Khelifi-Slaoui, M.; Amroune, S.; Benyoussef, E.H.; Thi, D.V.; Assaf-Ducrocq, C.; Gontier, E. Influence of minerals and elicitation on Datura stramonium L. tropane alkaloid production: Modelization of the in vitro biochemical response. Plant Sci. 2009, 177, 81–87. [Google Scholar] [CrossRef]
- Zhao, X.C.; Qu, X.; Mathews, D.E.; Schaller, G.E. Effect of ethylene pathway mutations upon expression of the ethylene receptor ETR1 from Arabidopsis. Plant Physiol. 2002, 130, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Parvatam, G.; Ravishankar, G.A. AgNO3—A potential regulator of ethylene activity and plant growth modulator. Electron. J. Biotechnol. 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Anantasaran, J.; Kanchanapoom, K. Influence of medium formula and silver nitrate on in vitro plant regeneration of Zinnia cultivars. Songklanakarin J. Sci. Technol. 2008, 30, 1–6. [Google Scholar]
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of plants. Biotechnol. Biotechnol. Equip. 2006, 20, 72–83. [Google Scholar] [CrossRef]
- Pitta–Alvarez, S.I.; Spollansky, T.C.; Giulietti, A.M. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzym. Microb. Technol. 2000, 26, 252–258. [Google Scholar] [CrossRef]
- Sahandi, S.; Sorooshzadeh, A.; Rezazadeh, H.S.; Naghdlbadl, H.A. Effect of nano silver and silver nitrate on seed yield of borage. J. Med. Plant. Res. 2011, 5, 706–710. [Google Scholar]
- Lee, W.M.; Kwak, J.I.; An, Y.J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere 2012, 86, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-G.; Xia, X.-X.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of cadaverine: A five carbon diamine. Biotechnol. Bioeng. 2011, 108, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Lili, M.; Wang, Y.; Chen, M.; Lan, X.; Wu, N.; Liao, Z. Effects of acetylsalicylic acid and UV-B on gene expression and tropane alkaloid biosynthesis in hairy root cultures of Anisodus luridus. Plant Cell Tissue Organ Cult. 2014, 117, 483–490. [Google Scholar] [CrossRef]
- Docimo, T.; Davis, A.J.; Luck, K.; Fellenberg, C.; Reichelt, M.; Phillips, M.; Gershenzon, J.; D’Auria, J.C. Influence of medium and elicitors on the production of cocaine, amino acids and phytohormones by Erythroxylum coca calli. Plant Cell Tissue Organ Cult. 2014, 120, 1061–1075. [Google Scholar] [CrossRef]
- Coste, A.; Vlase, L.; Halmagyi, A.; Deliu, C.; Coldea, G. Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Organ Cult. 2011, 106, 279–288. [Google Scholar] [CrossRef]
- Wurtzel, E.T.; Kutchan, T.M. Plant metabolism, the diverse chemistry set of the future. Science 2016, 353, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).