Synthesis, Characterization, and Anti-Inflammatory Activities of Methyl Salicylate Derivatives Bearing Piperazine Moiety
Abstract
:1. Introduction
2. Results
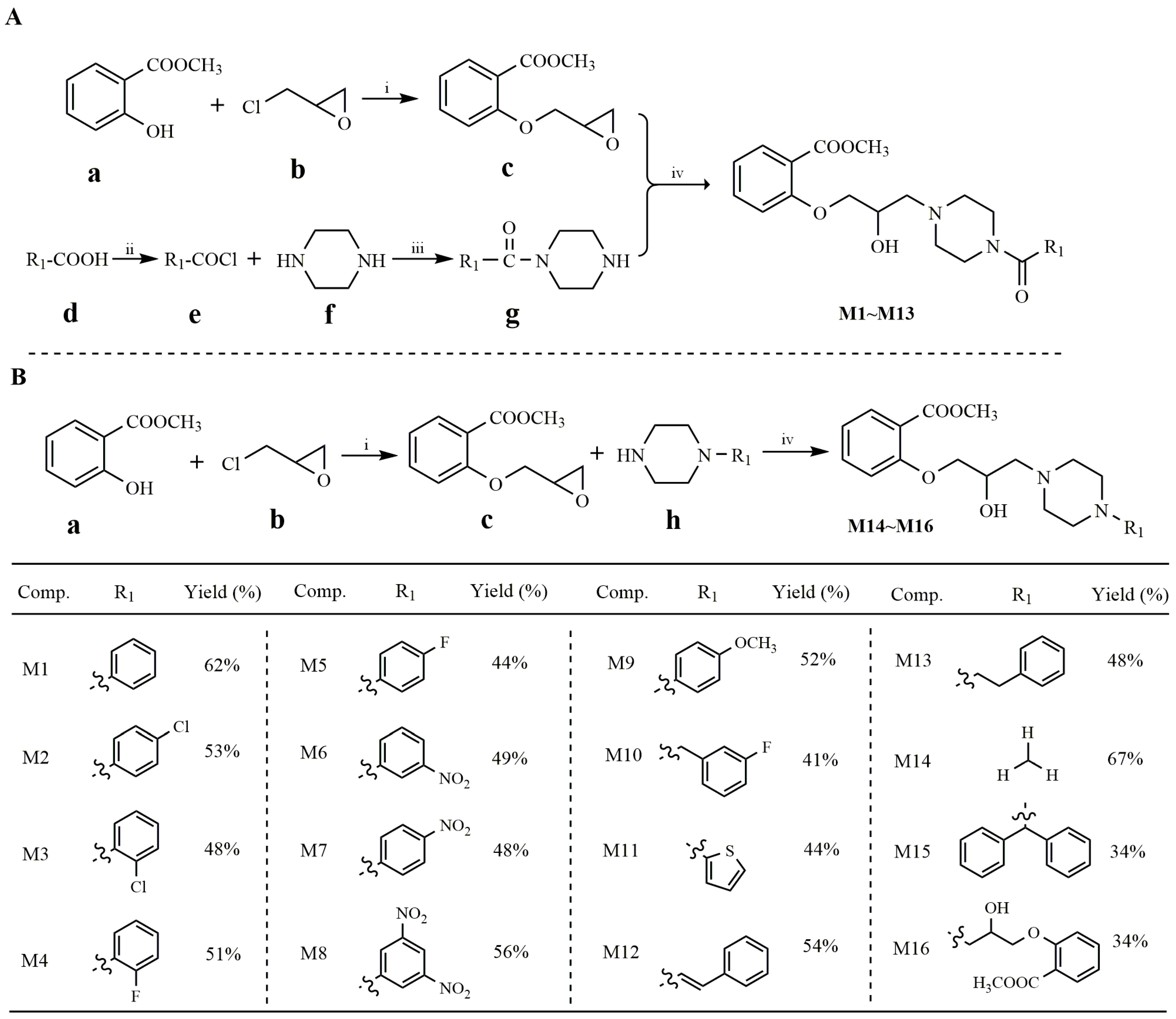
2.1. Synthesis of Salicylate Derivatives Bearing Piperazine Moiety
2.2. In Vivo Anti-Inflammatory Activities of Salicylate Derivatives Bearing Piperazine Moiety
2.3. Cytotoxicity Assays of Compounds M2, M14, M15, and M16
2.4. Inhibitory Screening against Lipopolysaccharide (LPS)-Induced Interleukin (IL)-6 and Tumor Necrosis Factor (TNF)-α Release
2.5. Compound M16 Attenuates LPS Induced Cyclooxygenase-2 (COX-2) Up-Regulation
3. Experimental Section
3.1. Synthetic Details of Intermediates and Target Compounds
3.2. Cell Culture and Cytotoxicity Assays
3.3. In Vivo Anti-Inflammatory Activity Assays in Ear and Paw Edema
3.4. ELISA Assay
3.5. Western Blot Assays
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, I. Pain market. Nat. Rev. Drug Discov. 2010, 9, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, C.A.; Chen, Q.H.; Citro, M.L.; Keefer, L.K.; Knaus, E.E. Second-generation aspirin and indomethacin prodrugs possessing an O2-(acetoxymethyl)-1-(2-carboxypyrrolidin-1-yl)diazenium-1,2-diolate nitric oxide donor moiety: Design, synthesis, biological evaluation, and nitric oxide release studies. J. Med. Chem. 2008, 51, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Dannhardt, G.; Kiefer, W.; Kramer, G.; Maehrlein, S.; Nowe, U.; Fiebich, B. The pyrrole moiety as a template for COX-1/COX-2 inhibitors. Eur. J. Med. Chem. 2000, 35, 499–510. [Google Scholar] [CrossRef]
- Dubost, J.J.; Soubrier, M.; Sauvezie, B. Treatment of rheumatoid polyarthritis: Evolution of concepts and strategies. Rev. Med. Interne 1999, 20, 171–178. [Google Scholar] [CrossRef]
- Uchoa Fde, T.; da Silva, T.G.; de Lima Mdo, C.; Galdino, S.L.; Pitta Ida, R.; Dalla Costa, T. Preclinical pharmacokinetic and pharmacodynamic evaluation of thiazolidinone PG15: An anti-inflammatory candidate. J. Pharm. Pharmacol. 2009, 61, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Nagarapu, L.; Mateti, J.; Gaikwad, H.K.; Bantu, R.; Sheeba Rani, M.; Prameela Subhashini, N.J. Synthesis and anti-inflammatory activity of some novel 3-phenyl-N-[3-(4-phenylpiperazin-1yl)propyl]-1H-pyrazole-5-carboxamide derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 4138–4140. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, S.; Melillo, G.; Bestetti, A.; Borsa, M.; Giuliani, P.; Tonon, G.C. Antitussive properties of levodropropizine. Arzneim. Forsch. 1988, 38, 1141–1143. [Google Scholar]
- Chen, Y.; Wang, G.; Xu, X.; Liu, B.F.; Li, J.; Zhang, G. Design, synthesis and biological activity evaluation of arylpiperazine derivatives for the treatment of neuropathic pain. Molecules 2011, 16, 5785–5806. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lim, H.D.; Zhang, M.; Desai, P.; Dai, H.; Colling, P.M.; Leurs, R.; Thurmond, R.L. Cloning and pharmacological characterization of the dog histamine H4 receptor. Eur. J. Pharmacol. 2008, 592, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Thurmond, R.L.; Desai, P.J.; Dunford, P.J.; Fung-Leung, W.P.; Hofstra, C.L.; Jiang, W.; Nguyen, S.; Riley, J.P.; Sun, S.; Williams, K.N.; et al. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J. Pharmacol. Exp. Ther. 2004, 309, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Liu, Z.; Xie, M.; Jiang, R.; Liu, W.; Wang, X.; Meng, S.; She, G. Naturally occurring methyl salicylate glycosides. Mini Rev. Med. Chem. 2014, 14, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Huang, C.; Zhang, X.; Xin, S.; Zhou, Y.; Ma, X.; Zhang, D.; Li, Y.; Zhou, S.; Zhang, D.; et al. Methyl salicylate lactoside inhibits inflammatory response of fibroblast-like synoviocytes and joint destruction in collagen-induced arthritis in mice. Br. J. Pharmacol. 2014, 171, 3526–3538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, J.; Xin, W.; Li, Y.; Ni, L.; Ma, X.; Zhang, D.; Zhang, D.; Zhang, T.; Du, G. Anti-inflammation effect of methyl salicylate 2-O-β-d-lactoside on adjuvant induced-arthritis rats and lipopolysaccharide (LPS)-treated murine macrophages RAW264.7 cells. Int. Immunopharmacol. 2015, 25, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Arbo, M.D.; Bastos, M.L.; Carmo, H.F. Piperazine compounds as drugs of abuse. Drug Alcohol. Depend. 2012, 122, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Maurer, H.H.; Kraemer, T.; Springer, D.; Staack, R.F. Chemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: A synopsis. Ther. Drug Monit. 2004, 26, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Drugs for parasitic infections. Med. Lett. Drugs Ther. 1993, 35, 111–122. [Google Scholar]
- Domagala, J.M. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J. Antimicrob. Chemother. 1994, 33, 685–706. [Google Scholar] [CrossRef] [PubMed]
- Corriveau, C.C.; Danner, R.L. Endotoxin as a therapeutic target in septic shock. Infect. Agents Dis. 1993, 2, 35–43. [Google Scholar] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes. Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- West, M.A.; Seatter, S.C.; Bellingham, J.; Clair, L. Mechanisms of reprogrammed macrophage endotoxin signal transduction after lipopolysaccharide pretreatment. Surgery 1995, 118, 220–228. [Google Scholar] [CrossRef]
- Yuan, C.; Rieke, C.J.; Rimon, G.; Wingerd, B.A.; Smith, W.L. Partnering between monomers of cyclooxygenase-2 homodimers. Proc. Natl. Acad. Sci. USA 2006, 103, 6142–6147. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for anti-iflammatory drugs. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds M2, M14, M15, and M16 are available from the authors.




| Compound | Dose (mg/kg) | Ear Edema | Paw Edema | Dose (mmol/kg) | ||
|---|---|---|---|---|---|---|
| Swelling Degree (mg) | Inhibition (%) | Swelling Degree (mg) | Inhibition (%) | |||
| Control | 8.36 ± 2.50 | - | 94.93 ± 8.46 | - | ||
| M1 | 100 | 2.82 ± 0.56 *** | 66.25 | 59.31 ± 6.25 *** | 37.52 | 0.25 |
| M2 | 100 | 2.29 ± 0.47 *** | 72.65 | 43.44 ± 8.65 *** | 54.24 | 0.23 |
| M3 | 100 | 2.42 ± 0.44 *** | 71.09 | 50.58 ± 7.40 *** | 46.72 | 0.23 |
| M4 | 100 | 2.70 ± 0.49 *** | 67.67 | 54.82 ± 5.03 ** | 42.25 | 0.24 |
| M5 | 100 | 4.61 ± 1.11 ** | 44.82 | 57.98 ± 4.94 ** | 38.92 | 0.24 |
| M6 | 100 | 4.54 ± 1.16 ** | 45.70 | 61.04 ± 5.58 *** | 35.70 | 0.23 |
| M7 | 100 | 5.03 ± 1.77 * | 39.80 | 64.58 ± 6.75 *** | 31.97 | 0.23 |
| M8 | 100 | 4.05 ± 1.55 ** | 51.55 | 59.60 ± 6.86 *** | 37.21 | 0.20 |
| M9 | 100 | 4.63 ± 1.43 ** | 44.65 | 59.22 ± 5.21 *** | 37.62 | 0.23 |
| M10 | 100 | 4.07 ± 1.08 ** | 51.30 | 79.22 ± 7.06 ** | 16.55 | 0.23 |
| M11 | 100 | 3.74 ± 1.13 *** | 55.31 | 49.24 ± 7.79 *** | 48.13 | 0.25 |
| M12 | 100 | 4.31 ± 1.37 ** | 48.40 | 59.91 ± 7.72 *** | 36.89 | 0.24 |
| M13 | 100 | 3.61 ± 1.17 *** | 56.80 | 53.43 ± 6.01 *** | 43.72 | 0.23 |
| M14 | 100 | 2.43 ± 0.75 *** | 70.97 | 43.64 ± 6.37 *** | 54.03 | 0.32 |
| M15 | 100 | 2.13 ± 1.09 *** | 74.48 | 41.42 ± 7.07 *** | 56.37 | 0.24 |
| M16 | 100 | 1.53 ± 0.55 *** | 81.73 | 33.43 ± 6.92 *** | 64.78 | 0.20 |
| Aspirin | 100 | 4.85 ± 1.70 * | 41.96 | 67.24 ± 8.68 *** | 29.16 | 0.56 |
| Indomethacin | 5 | 4.96 ± 1.09 ** | 40.66 | 64.13 ± 8.91 *** | 32.45 | 0.01 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Yin, Y.; Wang, L.; Liang, P.; Li, M.; Liu, X.; Wu, L.; Yang, H. Synthesis, Characterization, and Anti-Inflammatory Activities of Methyl Salicylate Derivatives Bearing Piperazine Moiety. Molecules 2016, 21, 1544. https://doi.org/10.3390/molecules21111544
Li J, Yin Y, Wang L, Liang P, Li M, Liu X, Wu L, Yang H. Synthesis, Characterization, and Anti-Inflammatory Activities of Methyl Salicylate Derivatives Bearing Piperazine Moiety. Molecules. 2016; 21(11):1544. https://doi.org/10.3390/molecules21111544
Chicago/Turabian StyleLi, Jingfen, Yong Yin, Lisheng Wang, Pengyun Liang, Menghua Li, Xu Liu, Lichuan Wu, and Hua Yang. 2016. "Synthesis, Characterization, and Anti-Inflammatory Activities of Methyl Salicylate Derivatives Bearing Piperazine Moiety" Molecules 21, no. 11: 1544. https://doi.org/10.3390/molecules21111544
APA StyleLi, J., Yin, Y., Wang, L., Liang, P., Li, M., Liu, X., Wu, L., & Yang, H. (2016). Synthesis, Characterization, and Anti-Inflammatory Activities of Methyl Salicylate Derivatives Bearing Piperazine Moiety. Molecules, 21(11), 1544. https://doi.org/10.3390/molecules21111544






