Effect of Thermoultrasound on the Antioxidant Compounds and Fatty Acid Profile of Blackberry (Rubus fruticosus spp.) Juice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Activity of Juices
2.1.1. Effect of Thermoultrasound and Pasteurization on the Ascorbic Acid Content
2.1.2. Effect of Thermoultrasound and Pasteurization on Total Phenolic and Anthocyanin Content
2.1.3. Effect of the Thermoultrasound and Pasteurization on the Antioxidant Activity of the Juices
2.2. Antioxidant Activity and Fatty Acid Profile of the n-Hexane Extracts from Blackberry Juice
2.2.1. Effect of Thermoultrasound and Pasteurization on the Antioxidant Activity of the Extracts
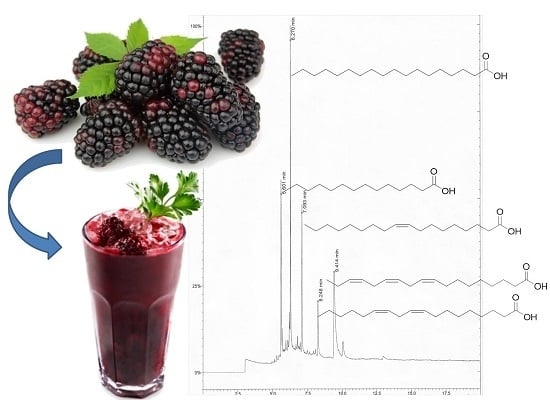
2.2.2. Fatty Acid Profile from Blackberry Juice
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Sample and Treatments
3.4. Determination of Ascorbic Acid Content
3.5. Determination of Total Phenolic Content
3.6. Determination of Anthocyanins
3.7. Antiradical Capacity by ABTS●+
3.8. Antiradical Capacity by DPPH●
3.9. Fatty Acids Profile by Gas Chromatography Coupled to Mass Spectroscopy
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lago-Vanzela, E.S.; Santos, G.V.; de Lima, F.A.; Gomes, E.; da-Silva, R. Physical-chemical, caloric and sensory characterization of light jambolan (Syzygium cumini Lamarck) jelly. Ciênc. Tecnol. Aliment. 2011, 31, 666–673. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Riaz, M.; de-Feo, V.; Jaafar, H.Z.E.; Moga, M. Rubus Fruticosus L.: Constituents, biological activities and health related uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Sorbitol, Rubus fruit, and misconception. Food Chem. 2015, 166, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Kaume, L.; Howard, L.; Devareddy, L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2011, 60, 5716–5727. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.; Guevara, A. Variación de la capacidad antioxidante y compuestos bioactivos durante el procesamiento del néctar de zarzamora (Rubus fructicosus L.). Rev. Soc. Quím. Per. 2013, 79, 116–125. [Google Scholar]
- Elizarrarás, A.C.; Martini, J.P.; Cansino, N.D.S.C. Optimización de las condiciones de termoultrasonicación del jugo de zarzamora sobre las características fisicoquímicas, microbiológicas y antioxidantes. Master’s Thesis, Universidad Autónoma del Estado de Hidalgo, Hidalgo, Mexico, 2015. [Google Scholar]
- Bushman, B.S.; Phillips, B.; Isbell, T.; Ou, B.; Crane, J.M.; Knapp, S.J. Chemical composition of cranberry (Rubus spp.) seeds and oils and their antioxidant potential. J. Agric. Food Chem. 2004, 52, 7982–7987. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Lima, G.P.; Vianello, F.; Corrêa, C.R.; Da-Silva-Campos, R.A.; Galhardo-Borguini, M. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr Sci. 2014, 5, 1065–1082. [Google Scholar] [CrossRef]
- Lado, B.H.; Yousef, A.E. Alternative food-preservation technologies: Efficacy and mechanisms. Microb. Infect. 2002, 4, 433–440. [Google Scholar] [CrossRef]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Shankar, R.; Kaushik, U.; A-Bhat, S. The Emerging technology in the sector of food technology-the non-thermal technology. IJIAS 2014, 6, 941–958. [Google Scholar]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Rojas, Q.Y.; Cruz-Cansino, N.; Ramírez-Moreno, E.; Delgado-Olivares, L.; Villanueva- Sánchez, J.; Alanís-García, E. Effects of ultrasoundtreatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason. Sonochem. 2013, 20, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonics in food processing, Trends. Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Dolatowski, Z.J.; Stadnik, J.; Stasiak, D. Applications of ultrasound in food technology. Acta Sci. Pol. Technol. Aliment. 2007, 6, 89–99. [Google Scholar]
- Valdramidis, V.P.; Cullen, P.J.; Tiwari, B.K.; O’Donnell, C.P. Quantitative modeling approaches for ascorbic acid degradation and non-enzymatic browning of orange juice during ultrasound processing. J. Food Eng. 2010, 9, 449–454. [Google Scholar] [CrossRef]
- Petrier, C.; Combet, E.; Mason, T. Oxygen-induced concurrent ultrasonic degradation of volatile and non-volatile aromatic compounds. Ultrason. Sonochem. 2007, 14, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Rawson, A.; Tiwari, B.K.; Patras, A.; Brunton, N.; Brennan, C.; Cullen, P.J.; O’Donnellc, C. Effect of thermosonication on bioactive compounds in watermelon juice. Food Res. Int. 2011, 44, 1168–1173. [Google Scholar] [CrossRef]
- Aguilar-Rosas, S.F.; Ballinas-Casarrubias, M.L.; Nevarez-Moorillon, G.V.; Martin-Belloso, O.; Ortega-Rivas, E. Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. J. Food Eng. 2007, 83, 41–46. [Google Scholar] [CrossRef]
- Cervantes-Elizarrarás, A.; Piloni-Martini, J.; Ramírez-Moreno, E.; Alanís-García, E.; Güemes-Vera, N.; Gómez-Aldapa, C.A.; Zafra-Rojas, Q.Y.; Cruz-Cansino, N.S. Enzymatic inactivation and antioxidant properties of blackberry juice after thermoultrasound: Optimization using response surface methodology. Ultrason. Sonochem. 2017, 34, 371–379. [Google Scholar]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Radocâj, O.; Vujasinovi, V.; Dimi, E.; Basi’c, Z. Blackberry (Rubus fruticosus L.) and raspberry (Rubus idaeus L.) seed oils extracted from dried press pomace after longterm frozen storage of berries can be used as functional food ingredients. Eur. J. Lipid Sci. Technol. 2014, 116, 1015–1024. [Google Scholar]
- Pingret, D.; Fabiano-Tixier, A.S.; le Bourvellec, C.; Renard, C.M.G.C.; Chemat, F. Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Grondin, I.; Costes, P.; Moutoussamy, L.; Shum, A.; Sing, A.S.C.; Smadja, J. High power ultrasound effects on lipid oxidation of refined sunflower oil. Ultrason. Sonochem. 2004, 11, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its component. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Tanaka, Y.; Endang, H.; Umar, M.; Satake, T. Chemical constituents of Morinda citrifolia fruits inhibit copper-induced low-density lipoprotein oxidation. J. Agric. Food. Chem. 2004, 52, 5843–5848. [Google Scholar] [CrossRef] [PubMed]
- Dürüst, N.; Sümengen, D.; Dürüst, Y. Ascorbic acid and element contents of foods of Trabzon (Turkey). J. Agric. Food Chem. 1997, 45, 2085–2087. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, Betalain Pattern, and Antioxidant Properties of Cactus Pear (Opuntia spp.) Clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Kuskoski, E.; AsueroI, A.G.; TroncosoI, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciênc. Tecnol. Aliment. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Morales, F.J.; Jimenez-Perez, S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. J. Agric. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.

| Determination | CTL | PAS | TUS |
|---|---|---|---|
| Ascorbic acid (mg AA/L) | 27.7 ± 0.8 c | 25.2 ± 0.8 b | 21.3 ± 0.6 a |
| Total phenols (mg GAE/L) | 726.2 ± 4.8 a | 789.6 ± 3.9 b | 1011.6 ± 3.9 c |
| Anthocyanins (mg Cy-3-GlE/L) | 106.3 ± 1.3 b | 94.4 ± 4.2 a | 118.7 ± 1.4 c |
| ABTS (mg VCEAC/L) | 13.6 ± 0.2 b | 11.5 ± 0.4 a | 44.7 ± 1.2 c |
| DPPH (µmol TE/L) | 1146.5 ± 1.9 a | 1319.8 ± 1.0 b | 2655.9 ± 14.0 c |
| Determination | CTL | PAS | TUS |
|---|---|---|---|
| ABTS (mg VCEAC/L) | 63.7 ± 1.1 b | 12.1 ± 1.0 a | 177.5 ± 0.7 c |
| DPPH (µmol TE/L) | 445.2 ± 1.6 b | 99.5 ± 3.0 a | 1802.6 ± 1.1 c |
| Fatty Acid (% w/w) | Blackberry Juice Oil | ||
|---|---|---|---|
| Control | Pasteurized | Thermoultrasonic | |
| C 14:0 | 21.8 ± 0.1 b | 23.8 ± 0.6 c | 18.0 ± 0.7 a |
| C 18:0 | 40.5 ± 0.3 c | 21.1 ± 0.4 a | 23.2 ± 0.4 b |
| C 18:1 | 19.1 ± 0.2 c | 14.6 ± 0.4 a | 14.7 ± 0.3 b |
| C 18:2n-6 | 7.7 ± 0.4 b | 7.3 ± 0.1 a | 7.8 ± 0.7 c |
| C 18:3n-3 | 11.0 ± 0.5 b | 14.9 ± 0.6 c | 7.6 ± 0.6 a |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manríquez-Torres, J.D.J.; Sánchez-Franco, J.A.; Ramírez-Moreno, E.; Cruz-Cansino, N.D.S.; Ariza-Ortega, J.A.; Torres-Valencia, J.M. Effect of Thermoultrasound on the Antioxidant Compounds and Fatty Acid Profile of Blackberry (Rubus fruticosus spp.) Juice. Molecules 2016, 21, 1624. https://doi.org/10.3390/molecules21121624
Manríquez-Torres JDJ, Sánchez-Franco JA, Ramírez-Moreno E, Cruz-Cansino NDS, Ariza-Ortega JA, Torres-Valencia JM. Effect of Thermoultrasound on the Antioxidant Compounds and Fatty Acid Profile of Blackberry (Rubus fruticosus spp.) Juice. Molecules. 2016; 21(12):1624. https://doi.org/10.3390/molecules21121624
Chicago/Turabian StyleManríquez-Torres, José De Jesús, José Antonio Sánchez-Franco, Esther Ramírez-Moreno, Nelly Del Socorro Cruz-Cansino, José Alberto Ariza-Ortega, and Jesús Martín Torres-Valencia. 2016. "Effect of Thermoultrasound on the Antioxidant Compounds and Fatty Acid Profile of Blackberry (Rubus fruticosus spp.) Juice" Molecules 21, no. 12: 1624. https://doi.org/10.3390/molecules21121624
APA StyleManríquez-Torres, J. D. J., Sánchez-Franco, J. A., Ramírez-Moreno, E., Cruz-Cansino, N. D. S., Ariza-Ortega, J. A., & Torres-Valencia, J. M. (2016). Effect of Thermoultrasound on the Antioxidant Compounds and Fatty Acid Profile of Blackberry (Rubus fruticosus spp.) Juice. Molecules, 21(12), 1624. https://doi.org/10.3390/molecules21121624