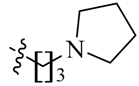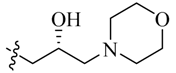Abstract
We report herein the design and synthesis of a series of novel 5-bromo-7-azaindolin-2-one derivatives containing a 2,4-dimethyl-1H-pyrrole-3-carboxamide moiety. These newly synthesized derivatives were evaluated for in vitro activity against selected cancer cell lines by MTT assay. Results revealed that some compounds exhibit broad-spectrum antitumor potency, and the most active compound 23p (IC50: 2.357–3.012 μM) was found more potent than Sunitinib (IC50: 31.594–49.036 μM) against HepG2, A549 and Skov-3, respectively.
1. Introduction
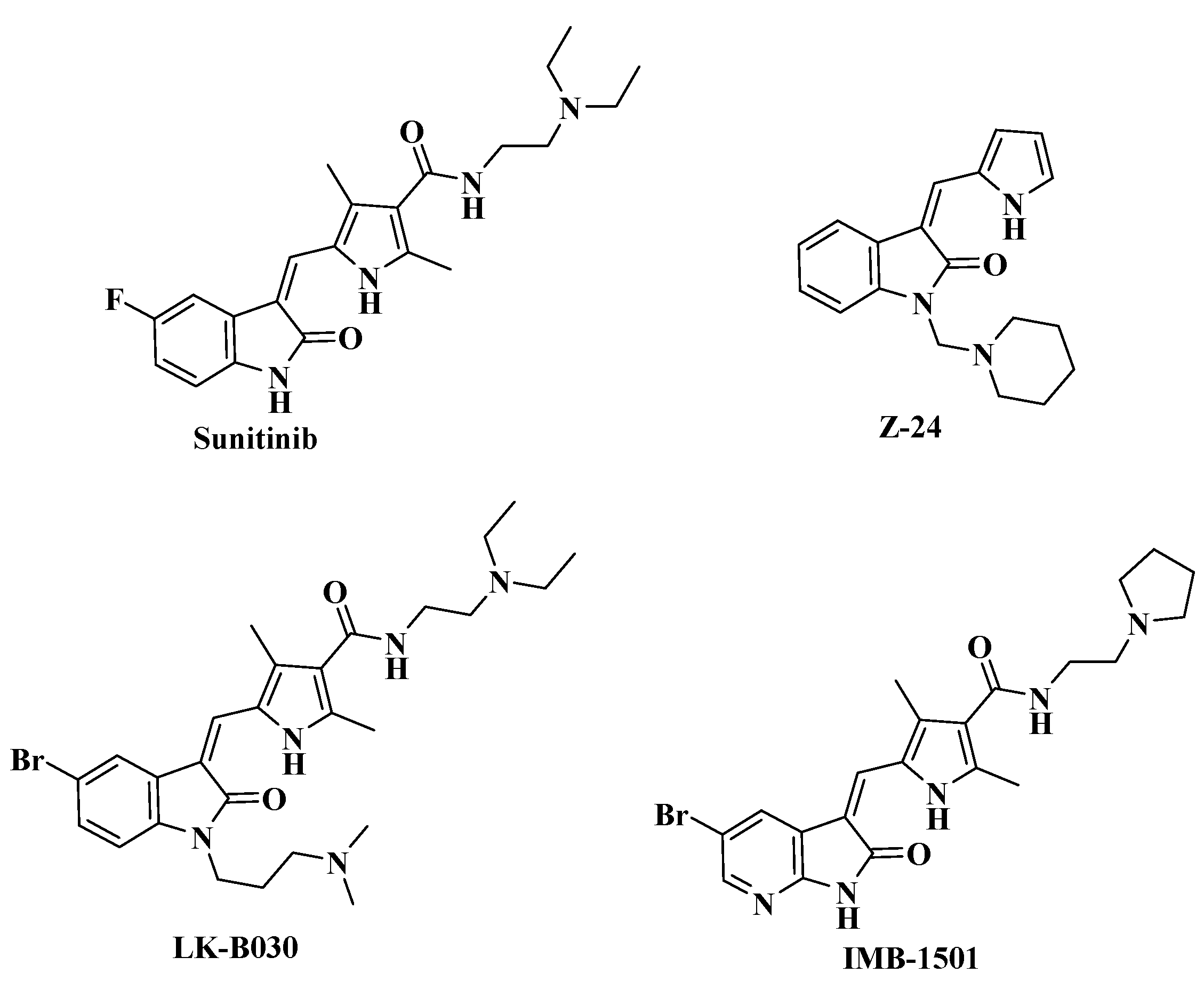
Sunitinib (Figure 1) is a new multitargeted oral anti-angiogenic and antitumor drug that has been recently approved against gastrointestinal stromal tumors (GIST) and advanced renal cell carcinoma (RCC) [1]. It is in clinical studies for the treatment of other solid tumors, such as pancreatic neuroendocrine tumors [2], meningioma [3], metastatic breast cancer [4] and non-small cell lung cancer [5].
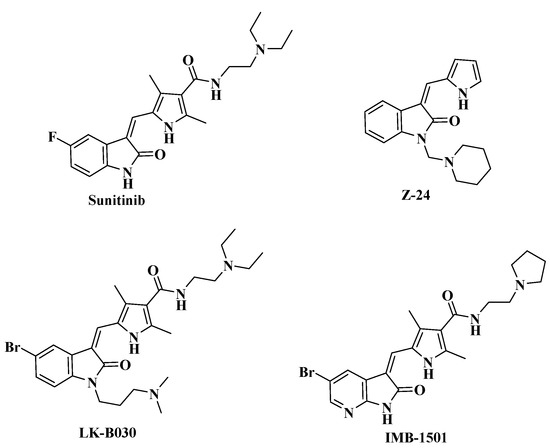
Figure 1.
Structures of Sunitinib, Z-24, LK-B030 and IMB-1501.
Recently, structural modifications mainly at the 3- and 5-positions of the indolin-2-one ring of Sunitinib have made considerable progress in the ability to increase antitumor activity through inhibition on different receptors [6,7,8]. As early lead compounds discovered in our lab, Z24 and LK-B030 (Figure 1) bearing a (piperidin-1-yl)methyl and a (3-dimethylamino)propyl group at the N-1 position, respectively, display a broad spectrum of antitumor activity by inhibiting angiogenesis in new blood vessels [9,10,11]. More recently, we reported a series of novel 5-halogenated-7-azaindolin-2-one derivatives and found IMB-1501 to have better in vitro activity than Sunitinib against the entire tested cancer cell lines [12].
As part of our continuing modifications on Sunitinib as a potential antitumor drug candidate, we planned to explore other possibilities for diversification of the 2-(pyrrolidin-1-yl)ethyl group and the linker flexibility on the amide bond. Thus, a series of novel 5-bromo-7-azaindolin-2-one derivatives containing a 2,4-dimethyl-1H-pyrrole-3-carboxamide moiety were designed, synthesized and evaluated for their antitumor activity in this study. Our primary objective was to optimize the potency of these compounds against a set of solid tumors and contribute to the development of new antitumor agents. A preliminary structure-activity relationship (SAR) study is also explored to facilitate the further development of 5-bromo-7-azaindolin-2-ones.
2. Results and Discussion
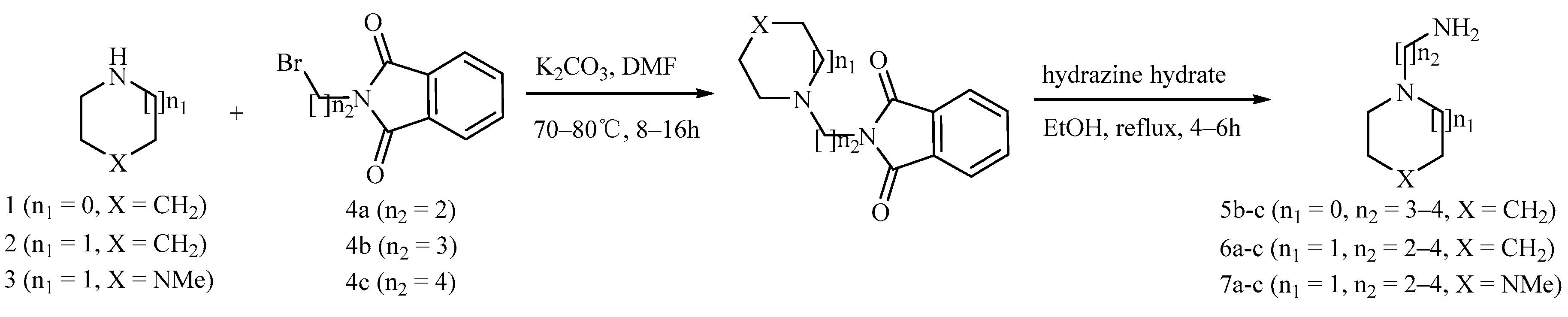
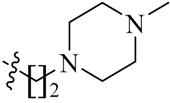
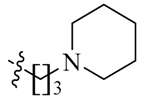
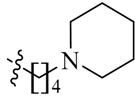
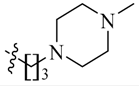
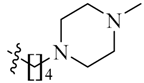
Detailed synthetic pathways to heterocyclic amine derivatives 5–7 and 14–16, which are commercially unavailable, and target compounds 23a–q are depicted in Scheme 1, Scheme 2 and Scheme 3, respectively. Amine derivatives containing aminoalkyl groups 5–7 were easily obtained from pyrrolidine 1, piperidine 2 and N-methylpiperazine 3 by nucleophilic substitution with N-(2-bromoethyl)/N-(3-bromopropyl)/N-(4-bromobutyl)phthalimides 4a–c in the presence of K2CO3 in DMF at 70–80 °C, respectively, followed by treatment of the resulting condensates with hydrazine hydrate in ethanol under reflux condition (Scheme 1).
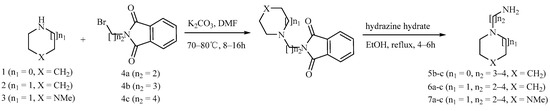
Scheme 1.
Synthesis of amine derivatives 5–7.
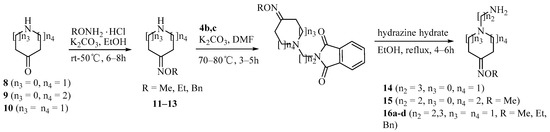
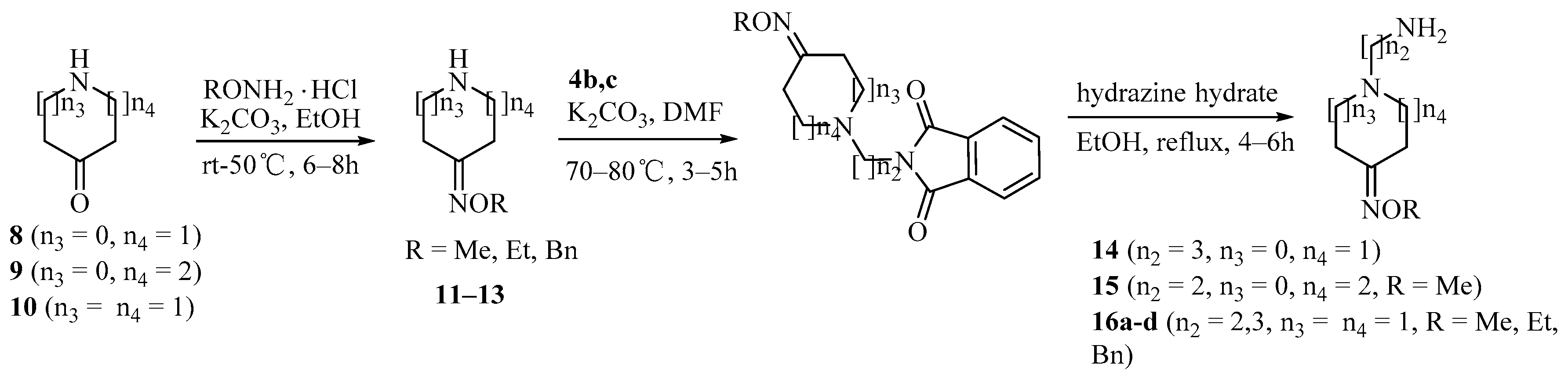
Scheme 2.
Synthesis of amine derivatives 14–16.
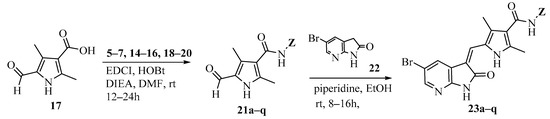
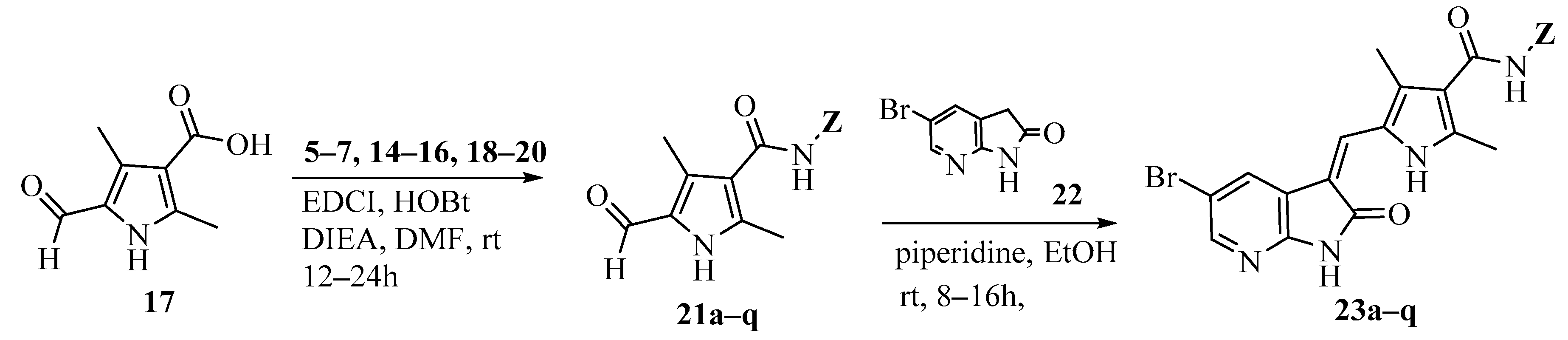
Scheme 3.
Synthesis of target compounds 23a–q.
Condensation of pyrrolidin-3-one 8, piperidin-3-one 9 and piperidin-4-one 10 with O-alkylhydroxyamines gave compounds 11–13. Amine derivatives 14–16 were prepared from oximes 11–13 by coupling with 4b,c and hydrazinolysis, sequentially (Scheme 2).
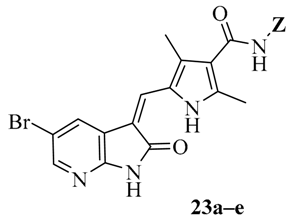
Amidation of 5-formyl-2,4-dimethylpyrrole-3-carboxylic acid 17 with 5–7, 14–16 and commercially available (S)-1-amino-3-morpholinopropan-2-ol (18), N1,N1-dimethylpropane-1,3-diamine (19) and N1,N1-diethylpropane-1,3-diamine (20) in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI), N-hydroxybenzotriazole (HOBt) and Ethyldiisopropylamine (DIEA) yielded compounds 21a–q. Aldol condensation of 5-formyl-2,4-dimethylpyrrole-3-carboxamides 21a–q with 5-bromo-7-azaindolin-2-one 23 in the presence of piperidine gave the target compounds 23a–q (Scheme 3) [10,11]. All of the new synthetic compounds were well characterized by 1H-NMR, 13C-NMR and MS. As expected, the pyrrole-2-methylidene geometry at the 3-position of the 7-azaindolin-2-one ring was confirmed to have the Z-configuration [12,13,14].
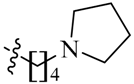
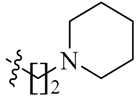
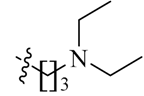
We first replaced the ethylidyne linker of Sunitinib with propylidyne or butylidyne, and the diethylamino group with a saturated heterocyclic amine (pyrrolidine, piperidine, piperazine, or hydroxylmorphine [15]) to synthesize the derivatives 23a–e. For preliminary screening of antitumor candidates, the target compounds were investigated for cytotoxic activity in vitro against MCF-7 (a breast cancer cell line). It is encouraging that all of the initially designed molecules except 23e exhibit higher inhibition (81.46%–87.29%) than Sunitinib (27.79%) at the concentration of 30 μM (Table 1). They were further evaluated for their in vitro antitumor activity in six human cancer cell lines, including MCF-7, HepG2 (liver carcinoma), HT-29 (colon adenocarcinoma), A549 (lung adenocarcinoma), PANC-1 (pancreatic carcinoma) and Skov-3 (ovarian carcinoma) by MTT assay [16].

Table 1.
In vitro activity of target compounds 23a–e against six cell lines.
The data reveal that 5-bromo-7-azaindolin-2-ones 23a–e demonstrate increased activity (IC50: 3.103–65.054 μM) compared to Sunitinib (IC50: 29.257–65.606 μM) against all of the tested cancer cell lines (Table 1). In particular, compound 23c exhibits a value of 3.103 μM against A549 and 23d exhibits a IC50 value of 3.721 μM against Skov-3, which are 9.4- and 8.6-fold more potent than Sunitinib, respectively. It seems likely that the linker between the amide and the pyridine ring is well tolerated with an alkyl chain of C3/C4 (23a–b). Moreover, bearing a pyridine or piperidine or piperazine moiety with an alkyl chain of C2–4 is more favorable than the introduction of a hydroxy group on the alkyl linker (23a–d vs. 23e).
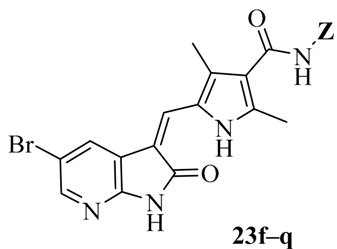
Being encouraged by the above results, we further explored other possibilities for diversification of the linker or/and heterocyclic amine to design and synthesize the derivatives 23f–q which were evaluated for their activity in selected cell lines HepG2, A549 and SKOV-3 (Table 2). When the ethylidyne linker (23c, 23d) was replaced by a propylidyne or butylidyne moiety, the resulting compounds (23f, 23g, 23i) were found to have better activity (IC50: 5.023–7.803 μM) than Sunitinib (IC50: 31.594–49.036 μM). However, replacement of the butylidyne linker (23i) with a propylidyne moiety or opening of the piperidine ring (23f) led to decreased potency, although the corresponding compounds (23h, 23j, 23k) demonstrate similar activity.

Table 2.
In vitro activity of target compounds 23f–q against three cell lines.
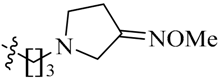
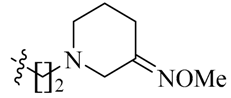
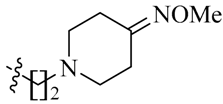
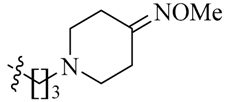
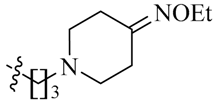
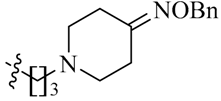
Considering the importance of an oxime functional moiety of the C-7 side chain with respect to the antibacterial and/or antitumor activity of quinolones [17,18,19], the impact of an alkoxyimino group on the pyrrolidine or piperidine ring was also investigated. It is clear that the introduction of a methoxyimino group on the pyrrolidine ring is detrimental (23l). For the piperidine ring, the nature and position of the oxime group and linker greatly influence activity. For instance, the presence of a methoxyimino group at the para-position is more favorable than the meta-position (23n vs. 23m). In addition, the propylidyne linker (23o) displays MIC values of 6.828–7.747 μM, which are 2.2- to 4.7-fold more potent than the ethylidyne linker (23n). It is also shown that the methyl group of the oxime moiety (23o) could be replaced by an ethyl (23p) or a benzyl (23q) one without obviously affecting the antitumor potency. Among the three, the most active compound 23p (IC50: 2.357–3.012 μM) was found to be 11.3- to 18.4-fold more potent than Sunitinib against all of the tested cell lines, respectively (Table 2).
3. Experimental Section
Melting points were determined in open capillaries and are uncorrected. 1H-NMR and 13C-NMR spectra were determined on a Varian Inova-500 spectrometer (Varian, Inc., Palo Alto, CA, USA) in DMSO-d6 using tetramethylsilane as an internal standard. Electrospray ionization (ESI) mass spectra were obtained on an MDSSCIEX Q-Tap mass spectrometer (AB Sciex, Redwood City, MA, USA) and Advion Mass Express 2.1.243 (Advion BioSciences, Inc., Ithaca, NY, USA). The reagents were all of analytical grade or chemically pure. TLC was performed on silica gel plates (Merck, ART5554 60F254, Kenilworth, NJ, USA).
A mixture of heterocyclic amines (1–3, 5.9 mmol), 2-(2-bromoethyl/propyl/butyl)isoindoline-1,3-diones (4a–c, 7.0 mmol) and potassium carbonate (7.0 mmol) in N,N-dimethylformamide (10 mL) was stirred for 8–16 h at 70–80 °C. Then the reaction mixture was cooled to temperature and was added water. The aqueous layer was extracted with dichloromethane (30 mL × 3) and the combined organic layer was concentrated under reduced pressure. The residue was solved in ethanol (10 mL) and treated with 80% hydrazine hydrate (10.5 mmol). The reaction mixture was stirred at reflux for 4–6 h and concentrated under reduced pressure to give the title compounds 5b–c, 6a–c and 7a–c as yellow oils or off-white solids.
A mixture of pyrrolidin-3-one/piperidin-3-one/piperidin-4-ones (8–10, 15.0 mmol), O-alkylhydroxyl amine hydrochlorides (18.0 mmol) and potassium carbonate (30.0 mmol) in ethanol (50 mL) was stirred for 6–8 h at 25–50 °C. Then the mixture was cooled to temperature and filtered, the filter cake washed with ethanol. The filtrate was concentrated under reduced pressure to afford the oximes 11–13 as oils. Amine derivatives 14, 15 and 16a–d were prepared from the oximes 11–13 by coupling with 4b,c and hydrazinolysis sequentially as described above.
A mixture of 5-formyl-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (17, 3.0 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDCI) (4.5 mmol) and HOBt (4.5 mmol) in N,N-Dimethylformamide (10 mL) was stirred for 0.5 h at room temperature. Then compounds 5–7, 14–16 or 18–20 (4.5 mmol) and DIEA (6.6 mmol) were added. The reaction mixture was stirred for 12–24 h and later water was added. The aqueous layer was extracted with dichloromethane/methanol (10:1, 30 mL × 2) and the combined organic layer was concentrated under reduced pressure. A solution of the residue and 5-bromo-1,3-dihydro-2H-pyrrolo[2,3-b]pyridin-2-one (22, 3.0 mmol) in ethanol (10 mL) was stirred at temperature for 8–16 h. The precipitate was filtered, purified via silica gel column chromatography (methanol/dichloromethane 40:1) and recrystallized from ethanol to afford the title compounds 23a–q (26%–33%, two steps) as yellow solids.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-2,4-dimethyl-N-[3-(pyrrolidin-1-yl)propyl]-1H-pyrrole-3-carboxamide (23a): Yield: 26%. m.p.: 222–224 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.44 (s, 1H), 11.63 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.74 (t, J = 5.5 Hz, 1H), 3.26 (q, J = 12.5 Hz, 2H), 2.46–2.47 (m, 6H), 2.44 (s, 3H), 2.42 (s, 3H), 1.65–1.71 (m, 6H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.4, 151.1, 144.2, 137.8, 131.8, 127.7, 126.7, 126.0, 122.2, 121.4, 112.5, 111.0, 53.5, 53.3, 52.0, 37.2, 28.0, 23.0, 13.4, 10.5, 7.2. MS-ESI (m/z): 472.6 (M + H)+, 474.6 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-2,4-dimethyl-N-[4-(pyrrolidin-1-yl)butyl]-1H-pyrrole-3-carboxamide (23b): Yield: 28%. m.p.: 230–232 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.44 (s, 1H), 11.63 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.74 (t, J = 5.5 Hz, 1H), 3.22 (q, J = 12.5 Hz, 2H), 2.43 (s, 3H), 2.41 (s, 3H), 2.37–2.40 (m, 6H), 1.65–1.67 (m, 4H), 1.48–1.53 (m, 4H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.3, 151.1, 144.1, 137.6, 131.8, 127.7, 126.8, 126.0, 122.3, 121.8, 112.5, 110.9, 55.4, 53.6, 38.6, 27.4, 26.0, 23.0, 13.3, 10.5. MS-ESI (m/z): 486.6 (M + H)+, 488.6 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-2,4-dimethyl-N-[2-(piperidin-1-yl)ethyl]-1H-pyrrole-3-carboxamide (23c): Yield: 28%. m.p.: 239–241 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.45 (s,1H), 11.63 (s, 1H), 8.48 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.86 (s, 1H), 7.52 (t, J = 5.5 Hz, 1H), 3.34 (t, J = 6.5 Hz, 2H), 2.46 (s, 3H), 2.44–2.45 (m, 6H), 2.44 (s, 3H), 2.17–2.36 (m, 6H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.3, 151.1, 144.2, 138.0, 131.8, 127.8, 126.7, 126.0, 122.2, 121.2, 112.5, 111.1, 57.2, 53.7, 52.0, 35.9, 25.3, 23.7, 13.4, 10.6, 7.2. MS-ESI (m/z): 472.6 (M + H)+, 474.6 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-2,4-dimethyl-N-[2-(4-methylpiperazin-1-yl)ethyl]-1H-pyrrole-3-carboxamide (23d): Yield: 32%. m.p.: 244–246 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.24 (s, 1H), 9.48 (s, 1H), 8.14 (d, J = 2.0 Hz, 1H), 7.77 (d, J = 2.0 Hz, 1H), 7.26 (s, 1H), 6.54 (t, J = 5.5 Hz, 1H), 3.55–3.58 (m, 2H), 2.64–2.66 (m, 4H), 2.58 (s, 3H), 2.43–2.50 (m, 4H), 2.37 (s, 3H), 2.30 (s, 3H), 1.60–1.64 (m, 2H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.2, 151.1, 144.1, 137.9, 131.7, 127.7, 126.7, 126.0, 122.2, 121.3, 112.5, 111.0, 56.7, 54.8, 52.5, 45.8, 36.1, 13.3, 10.6. MS-ESI (m/z): 487.7 (M + H)+, 489.7 (M + H)+.
(S,Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-N-(2-hydroxy-3-morpholinopropyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide (23e): Yield: 25%. m.p.: 284–286 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.45 (s, 1H), 11.64 (s, 1H), 8.48 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.86 (s, 1H), 7.61 (t, J = 5.5 Hz, 1H), 4.76 (s, 1H), 3.78–3.80 (m, 1H), 3.55–3.59 (m, 4H), 3.13–3.18 (m, 2H), 2.46 (s, 3H), 2.44 (s, 3H), 2.27–2.36 (m, 6H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.6, 151.1, 144.2, 137.9, 131.9, 127.7, 126.7, 126.0, 122.2, 121.3, 112.5, 111.0, 66.7, 66.2, 63.0, 54.0, 43.9, 13.4, 10.6 ppm. MS-ESI (m/z): 504.6 (M + H)+, 506.6 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-2,4-dimethyl-N-[3-(piperidin-1-yl)propyl]-1H-pyrrole-3-carboxamide (23f): Yield: 27%. m.p.: 236–238 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.44 (s, 1H), 11.64 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.56 (t, J = 6.5 Hz, 1H), 3.24 (q, J = 12.5 Hz, 2H), 2.44 (s, 3H), 2.42 (s, 3H), 2.17–2.37 (m, 6H), 1.38–1.67 (m, 8H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.4, 151.1, 144.1, 137.7, 131.8, 127.7, 126.7, 126.0, 122.2, 121.6, 112.5, 111.0, 56.3, 54.0, 37.3, 26.5, 25.4, 24.0, 13.3, 10.5 ppm. MS-ESI (m/z): 486.0 (M + H)+, 488.0 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-2,4-dimethyl-N-[4-(piperidin-1-yl)butyl]-1H-pyrrole-3-carboxamide (23g): Yield: 33%. m.p.: 238–240 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.44 (s, 1H), 11.64 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.73 (t, J = 5.5 Hz, 1H), 3.22 (q, J = 12.0 Hz, 2H), 2.43 (s, 3H), 2.41 (s, 3H), 2.22–2.37 (m, 6H), 1.43–1.53(m, 8H), 1.33–1.40 (m, 2H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.4, 151.1, 144.1, 137.6, 131.8, 127.7, 126.7, 126.0, 122.2, 121.8, 112.5, 110.9, 58.2, 54.0, 38.6, 27.3, 25.4, 24.1, 23.8, 13.2, 10.4 ppm. MS-ESI (m/z): 500.3 (M + H)+, 502.3 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-2,4-dimethyl-N-[3-(4-methylpiperazin-1-yl)propyl]-1H-pyrrole-3-carboxamide (23h): Yield: 30%. m.p.: 238–240 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.44 (s, 1H), 11.62 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.71 (t, J = 5.5 Hz, 1H), 3.22 (q, J = 12.5 Hz, 2H), 2.44 (s, 3H), 2.42 (s, 3H), 2.22–2.35 (m, 10H), 2.13 (s, 3H), 1.62–1.68 (m, 2H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.4, 151.1, 144.2, 137.7, 131.8, 127.8, 126.8, 126.0, 122.3, 121.7, 112.6, 111.0, 55.7, 54.7, 52.7, 45.7, 37.3, 26.6, 13.3, 10.5 ppm. MS-ESI (m/z): 501.3 (M + H)+, 503.3 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-2,4-dimethyl-N-[4-(4-methylpiperazin-1-yl)butyl]-1H-pyrrole-3-carboxamide (23i): Yield: 31%. m.p.: 238–240 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.43 (s, 1H), 11.63 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.73 (t, J = 5.5 Hz, 1H), 3.22 (q, J = 12.0 Hz, 2H), 2.43 (s, 3H), 2.41 (s, 3H), 2.16–2.37 (m, 10H), 2.13 (s, 3H), 1.45–1.50 (m, 4H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.4, 151.1, 144.1, 137.6, 131.8, 127.7, 126.8, 126.0, 122.3, 121.8, 112.5, 110.9, 57.6, 54.8, 52.7, 45.7, 38.6, 27.3, 23.9, 13.3, 10.5 ppm. MS-ESI (m/z): 515.3 (M + H)+, 517.3 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-N-[3-(dimethylamino)propyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (23j): Yield: 29%. m.p.: 258–260 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.44 (s, 1H), 11.61 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.73 (t, J = 5.5 Hz, 1H), 3.25 (q, J = 12.5 Hz, 2H), 2.45 (s, 3H), 2.42 (s, 3H), 2.30 (t, J = 6.5 Hz, 2H), 2.16 (s, 6H), 1.62–1.68 (m, 2H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.3, 151.1, 144.1, 137.8, 131.8, 127.7, 126.7, 126.0, 122.2, 121.5, 112.5, 111.0, 57.1, 45.2, 37.3, 27.1, 13.3, 10.5 ppm. MS-ESI (m/z): 446.2 (M + H)+, 448.2 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-N-[3-(diethylamino)propyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (23k): Yield: 30%. m.p.: 234–236 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.45 (s, 1H), 11.63 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.11 (d, J = 2.0 Hz, 1H), 7.86 (s, 1H), 7.74 (t, J = 5.5 Hz, 1H), 3.25–3.31 (m, 2H), 3.06–3.15 (m, 6H), 2.47 (s, 3H), 2.44 (s, 3H), 1.86–1.92 (m, 2H), 1.21 (t, J = 7.2 Hz, 6H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.8, 151.2, 144.2, 137.9, 131.8, 127.8, 126.8, 126.0, 122.2, 121.1, 112.5, 111.2, 48.4, 46.0, 36.0, 23.5, 13.4, 10.6, 8.4 ppm. MS-ESI (m/z): 474.3 (M + H)+, 476.3 (M + H)+.
5-[(Z)-(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-N-{3-[(E)-3-(methoxyimino)pyrrolidin-1-yl]propyl}-2,4-dimethyl-1H-pyrrole-3-carboxamide (23l): Yield: 27%. m.p.: 226–228 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.45 (s, 1H), 11.63 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.75 (t, J = 5.5 Hz, 1H), 3.95–4.10 (m, 4H), 3.78 (s, 3H), 3.48–3.56 (m, 2H), 3.28–3.32 (m, 2H), 2.63–2.69 (m, 2H), 2.44 (s, 3H), 2.42 (s, 3H), 1.80–1.85 (m, 2H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.5, 154.1, 151.1, 144.2, 137.7, 131.8, 127.7, 126.7, 126.0, 122.2, 121.4, 112.5, 111.0, 62.7, 61.4, 45.6, 43.8, 35.6, 28.9, 18.5, 13.3, 10.5 ppm. MS-ESI (m/z): 515.1 (M + H)+, 517.1 (M + H)+.
5-[(Z)-(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-N-{2-[(E)-3-(methoxyimino)piperidin-1-yl]ethyl}-2,4-dimethyl-1H-pyrrole-3-carboxamide (23m): Yield: 26%. m.p.: 198–200 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.45 (s, 1H), 11.61 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.80 (t, J = 5.5 Hz, 1H), 3.98–4.24 (m, 4H), 3.74 (s, 3H), 3.46–3.48 (m, 4H), 2.44 (s, 3H), 2.42 (s, 3H), 2.22–2.36 (m, 2H), 1.63–1.69 (m, 2H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.6, 153.9, 151.1, 144.2, 137.8, 131.9, 127.8, 126.8, 126.0, 122.2, 121.2, 112.5, 111.1, 63.8, 61.0, 56.0, 43.3, 38.2, 22.8, 18.5, 13.3, 10.4 ppm. MS-ESI (m/z): 515.3 (M + H)+, 517.3 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-N-{2-[4-(methoxyimino)piperidin-1-yl]ethyl}-2,4-dimethyl-1H-pyrrole-3-carboxamide (23n): Yield: 29%. m.p.: 262–264 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.44 (s, 1H), 11.62 (s, 1H), 8.46 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.58 (t, J = 5.5 Hz, 1H), 3.71 (s, 3H), 3.33–3.39 (m, 2H), 2.56 (t, J = 6.0 Hz, 2H), 2.47–2.52 (m, 6H), 2.46 (s, 3H), 2.44 (s, 3H), 2.23 (t, J = 6.0 Hz, 2H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.2, 156.3, 151.1, 144.2, 137.9, 131.8, 127.7, 126.7, 126.0, 122.2, 121.4, 112.5, 111.0, 60.6, 56.2, 53.1, 51.7, 36.4, 30.8, 24.9, 13.3, 10.5 ppm. MS-ESI (m/z): 515.2 (M + H)+, 517.3 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-N-{3-[4-(methoxyimino)piperidin-1-yl]propyl}-2,4-dimethyl-1H-pyrrole-3-carboxamide (23o): Yield: 32%. m.p.: 233–235 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.44 (s, 1H), 11.63 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.71 (t, J = 5.5 Hz, 1H), 3.71 (s, 3H), 3.25 (q, J = 12.5 Hz, 2H), 2.45–2.49 (m, 6H), 2.44 (s, 3H), 2.42 (s, 3H), 2.38 (t, J = 5.9 Hz, 2H), 2.22 (t, J = 7.0 Hz, 2H), 1.65–1.71 (m, 2H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.4, 156.4, 151.1, 144.2, 137.7, 131.8, 127.7, 126.8, 126.0, 122.2, 121.6, 112.5, 111.0, 60.5, 55.0, 53.3, 52.0, 37.2, 30.7, 26.9, 24.8, 13.3, 10.5 ppm. MS-ESI (m/z): 529.3 (M + H)+, 531.3 (M + H)+.
(Z)-5-[(5-Bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl]-N-{3-[4-(ethoxyimino)piperidin-1-yl]propyl}-2,4-dimethyl-1H-pyrrole-3-carboxamide (23p): Yield: 31%. m.p.: 237–239 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.44 (s, 1H), 11.63 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.71 (t, J = 5.5 Hz, 1H), 3.97 (q, J = 7.0 Hz, 2H), 3.25 (q, J = 12.5 Hz, 2H), 2.45–2.48 (m, 6H), 2.44 (s, 3H), 2.42 (s, 3H), 2.37–2.40 (m, 2H), 2.22–2.24 (m, 2H), 1.65–1.71 (m, 2H), 1.16 (t, J = 7.0 Hz, 3H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.4, 156.0, 151.1, 144.1, 137.7, 131.8, 127.7, 126.7, 126.0, 122.2, 121.6, 112.5, 111.0, 67.8, 55.0, 53.4, 52.0, 37.2, 30.8, 26.9, 24.9, 14.5, 13.3, 10.5 ppm. MS-ESI (m/z): 543.3 (M + H)+, 545.3 (M + H)+.
(Z)-N-{3-[4-(Benzyloxyimino)piperidin-1-yl]propyl}-5-[(5-bromo-2-oxo-1,2-dihydro-3H-pyrrolo[2,3-b]pyridin-3-ylidene)methyl)]-2,4-dimethyl-1H-pyrrole-3-carboxamide (23q): Yield: 30%. m.p.: 226–228 °C. 1H-NMR (500 MHz, DMSO-d6) δ: 13.44 (s, 1H), 11.63 (s, 1H), 8.47 (d, J = 2.0 Hz, 1H), 8.10 (d, J = 2.0 Hz, 1H), 7.85 (s, 1H), 7.71 (t, J = 5.5 Hz, 1H), 7.27–7.36 (m, 5H), 4.99 (s, 2H), 3.25 (q, J = 12.5 Hz, 2H), 2.51–2.53 (m, 2H), 2.47–2.48 (m, 4H), 2.44 (s, 3H), 2.42 (s, 3H), 2.37–2.40 (m, 2H), 2.22 (t, J = 5.8 Hz, 2H), 1.65–1.71 (m, 2H) ppm. 13C-NMR (400 MHz, DMSO-d6) δ: 169.1, 164.4, 157.2, 151.1, 144.1, 138.2, 137.7, 131.8, 128.2, 127.7, 127.5, 126.7, 126.0, 122.2, 121.6, 112.5, 111.0, 74.3, 55.0, 53.3, 52.0, 37.2, 30.8, 26.9, 25.0, 13.3, 10.5 ppm. MS-ESI (m/z): 605.3(M + H)+, 607.3 (M + H)+.
4. Conclusions
In summary, a series of novel 5-bromo-7-azaindolin-2-one derivatives containing a 2,4-dimethyl-1H-pyrrole-3-carboxamide moiety were designed, synthesized and evaluated for their in vitro antitumor activity by MTT assay. Our results reveal that many target compounds exhibit broad-spectrum antitumor potency which is better than Sunitinib. The most active compound 23p (IC50: 2.357–3.012 μM) was found 11.3- to 8.4-fold more potent than Sunitinib against all of the tested cell lines, HepG2, A549 and Skov-3, respectively. Studies to determine the in vivo pharmacokinetics and efficacy of compounds 23c, 23d and 23p against some selected tumor cell lines are currently underway.
Acknowledgments
This work was supported by National Key Research and Development Program (2016YFA0201500), the National S&T Major Special Project on Major New Drug Innovations (No. 162 2014ZX09507009-003, No. 2015ZX09102007-008) and NSFC (No. 163 81373267, No. 21502237).
Author Contributions
Conceived of and designed the experiments: Yun Chai, Huiyuan Guo, and Mingliang Liu. Performed the experiments: Jun Zhang, Weiyi Shen and Xiaoning Li. Analyzed the data: Senjun Li and Kai Lv. Wrote the paper: Yun Chai, Mingliang Liu and Jun Zhang. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kassem, M.G.; Motiur Rahman, A.F.M.; Korashy, H.M. Sunitinib malate. Profiles Drug Subst. Excip. Relat. Methodol. 2012, 37, 363–388. [Google Scholar] [PubMed]
- Kulke, M.H.; Bendell, J.; Kvols, L.; Picus, J.; Pommier, R.; Yao, J. Evolving Diagnostic and Treatment Strategies for Pancreatic Neuroendocrine Tumors. J. Hematol. Oncol. 2011, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Drappatz, J.; Wen, P.Y. Targeted drug therapy for meningiomas. Neurosurg. Focus 2007, 23, E12. [Google Scholar] [CrossRef] [PubMed]
- Houk, B.E.; Bello, C.L.; Poland, B.; Rosen, L.S.; Demetri, G.D.; Motzer, R.J. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: Results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother. Pharmacol. 2010, 66, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Scagliotti, G.V.; Rosell, R.; Socinski, M.A.; Brahmer, J.; Atkins, J.; Pallares, C.; Burgess, R.; Tye, L.; Selaru, P.; et al. Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br. J. Cancer 2009, 101, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Islam, I.; Bryant, J.; Chou, Y.L.; Kochanny, M.J.; Lee, W.; Phillips, G.B.; Yu, H.Y.; Adler, M.; Whitlow, M.; Ho, E.; et al. Indolinone based phosphoinositide-dependent kinase-1 (PDK1) inhibitors. Part 1: Design, synthesis and biological activity. Bioorg. Med. Chem. Lett. 2007, 17, 3814–3818. [Google Scholar] [CrossRef] [PubMed]
- Islam, I.I.; Brown, G.; Bryant, J.; Hrvatin, P.; Kochanny, M.J.; Phillips, G.B.; Yuan, S.D.; Adler, M.; Whitlow, M.; Lentz, D.; et al. Indolinone based phosphoinositide-dependent kinase-1 (PDK1) inhibitors. Part 2: Optimization of BX-517. Bioorg. Med. Chem. Lett. 2007, 17, 3819–3825. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Su, Y.D.; Feng, J.; Fu, J.H.; Yang, J.L.; Xiao, L.; Peng, J.H.; Li, Y.L.; Zhang, L.; Hu, B.; et al. Novel potent orally active multitargeted receptor tyrosine kinase inhibitors: Synthesis, structure-activity relationships, and antitumor activities of 2-indolinone derivatives. J. Med. Chem. 2010, 53, 8140–8149. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Lin, C.; Zheng, Z.B.; Li, S.; Guo, S.X.; Zhang, X.Y.; Guo, S.; Zhang, X.Y.; Fu, M.; Liang, X.; et al. Angiogenesis Inhibitor Z24 Induces Endothelial Cell Apoptosis and Suppresses Tumor Growth and Metastasis. J. Pharmacol. Sci. 2005, 97, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liang, C.; Shirazian, S.; Zhou, Y.; Miller, T.; Cui, J.; Fukuda, J.Y.; Chu, J.Y.; Nematalla, A.; Wang, X.Y.; et al. Discovery of 5-[5-Fluoro-2-oxo-1,2-dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic Acid (2-Diethylaminoethyl)amide, a Novel Tyrosine Kinase Inhibitor Targeting Vascular Endothelial and Platelet-Derived Growth Factor Receptor Tyrosine Kinase. J. Med. Chem. 2003, 46, 1116–1119. [Google Scholar] [PubMed]
- Manley, J.M.; Kalman, M.J.; Conway, B.G.; Ball, C.C.; Havens, J.L.; Vaidyannathan, R. Early amidation approach to 3-[(4-amido)pyrrol-2-yl]-2-indolinones. J. Med. Chem. 2003, 68, 6447–6450. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Wang, L.L.; Liu, M.L.; Zhou, X.B.; Fan, S.Y.; Liu, H.Y.; Zheng, Z.B.; Li, S. Synthesis and antitumor activity of 5-[1-(3-(dimethylamino)propyl)-5-halogenated-2-oxoindolin-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxamides. Bioorg. Med. Chem. Lett. 2011, 21, 3062–3065. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Wang, L.L.; Zhou, X.B.; Liu, M.L.; Liu, H.Y.; Zheng, Z.B.; Li, S. Synthesis and in vitro antitumor activity of 1-(3-dimethylamino)propyl indolin-2-one derivatives. Med. Chem. Res. 2013, 22, 1723–1729. [Google Scholar] [CrossRef]
- Wang, M.; Ye, C.; Liu, M.L.; Wu, Z.Y.; Li, L.H.; Wang, C.L.; Liu, X.J.; Guo, H.Y. Synthesis and antitumor activity of 5-(5-halogenated-2-oxo-1H-pyrrolo[2,3-b]pyridin-(3Z)-ylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-carboxamides. Bioorg. Med. Chem. Lett. 2015, 25, 2782–2787. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, B.X.; Wu, T.; Wan, J.T.; Tu, Z.C.; Njire, M.; Wan, B.J.; Franzblauc, S.G.; Zhang, T.Y.; Lu, X.Y.; et al. Design, Synthesis, and Biological Evaluation of Pyrazolo[1,5-a]pyridine-3-carboxamides as Novel Antitubercular Agents. ACS Med. Chem. Lett. 2015, 6, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Green, L.M.; Reade, J.L.; Ware, C.F. Rapid colormetric assay for cell viability: Application to the quantitation of cytotoxic and growth inhibitory lymphokines. J. Immunol. Methods 1984, 70, 257–268. [Google Scholar] [CrossRef]
- Lv, K.; Sun, Y.X.; Sun, L.Y.; Wei, Z.Q.; Guo, H.Y.; Wu, J.W.; Liu, H.Y. Design, Synthesis, and in vitro Antibacterial Activity of Fluoroquinolone Derivatives Containing a Chiral 3-(Alkoxyimino)-2-(aminomethyl)azetidine Moiety. ChemMedChem 2012, 7, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Guo, Q.; Wang, Y.C.; Liu, B.Q.; Liu, M.L.; Sun, L.Y.; Guo, H.Y. Synthesis and antibacterial activity of 7-(3-amino-4-alkoxyimino-1-piperidyl)-quinolones. Acta Pharm. Sin. 2008, 43, 819–827. [Google Scholar]
- Jia, X.D.; Wang, S.; Wang, M.H.; Liu, M.L.; Xia, G.M.; Liu, X.J.; Chai, Y.; He, H.W. Synthesis and in vitro antitumor activity of novel naphthyridinone derivatives. Chin. Chem. Lett. 2016. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 23f–q are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).