Scoparone Inhibits LPS-Simulated Inflammatory Response by Suppressing IRF3 and ERK in BV-2 Microglial Cells
Abstract
:1. Introduction
2. Results
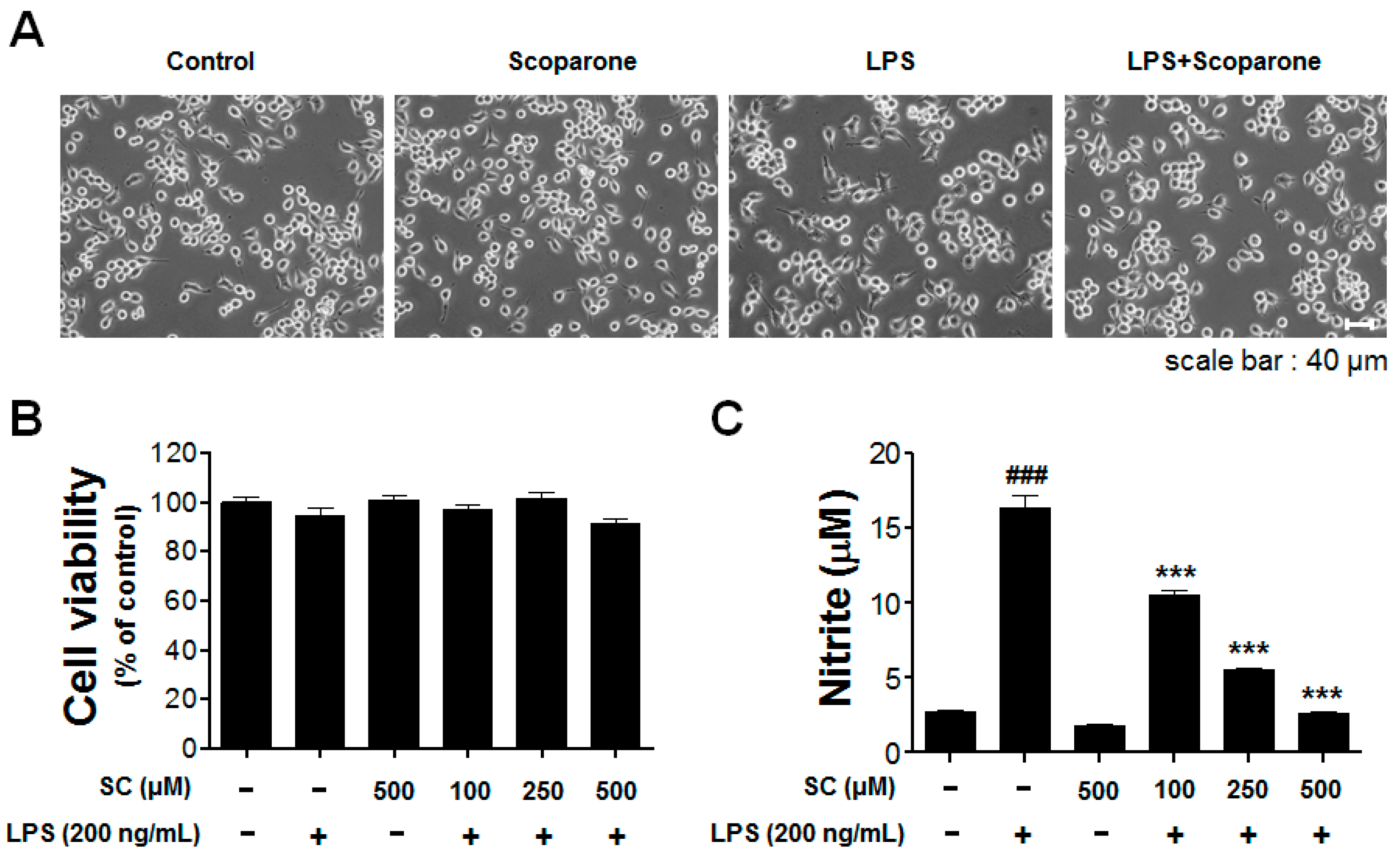
2.1. Effect of Scoparone on LPS-Induced Cellular Viability and Nitrite Production in BV-2 Microglial Cells
2.2. Effect of Scoparone on Levels of iNOS-mRNA Expression and Protein Induction in BV-2 Microglial Cells
2.3. Effect of Scoparone on COX-2-mRNA Expression and Protein Induction on LPS-Induced BV-2 Microglial Cells
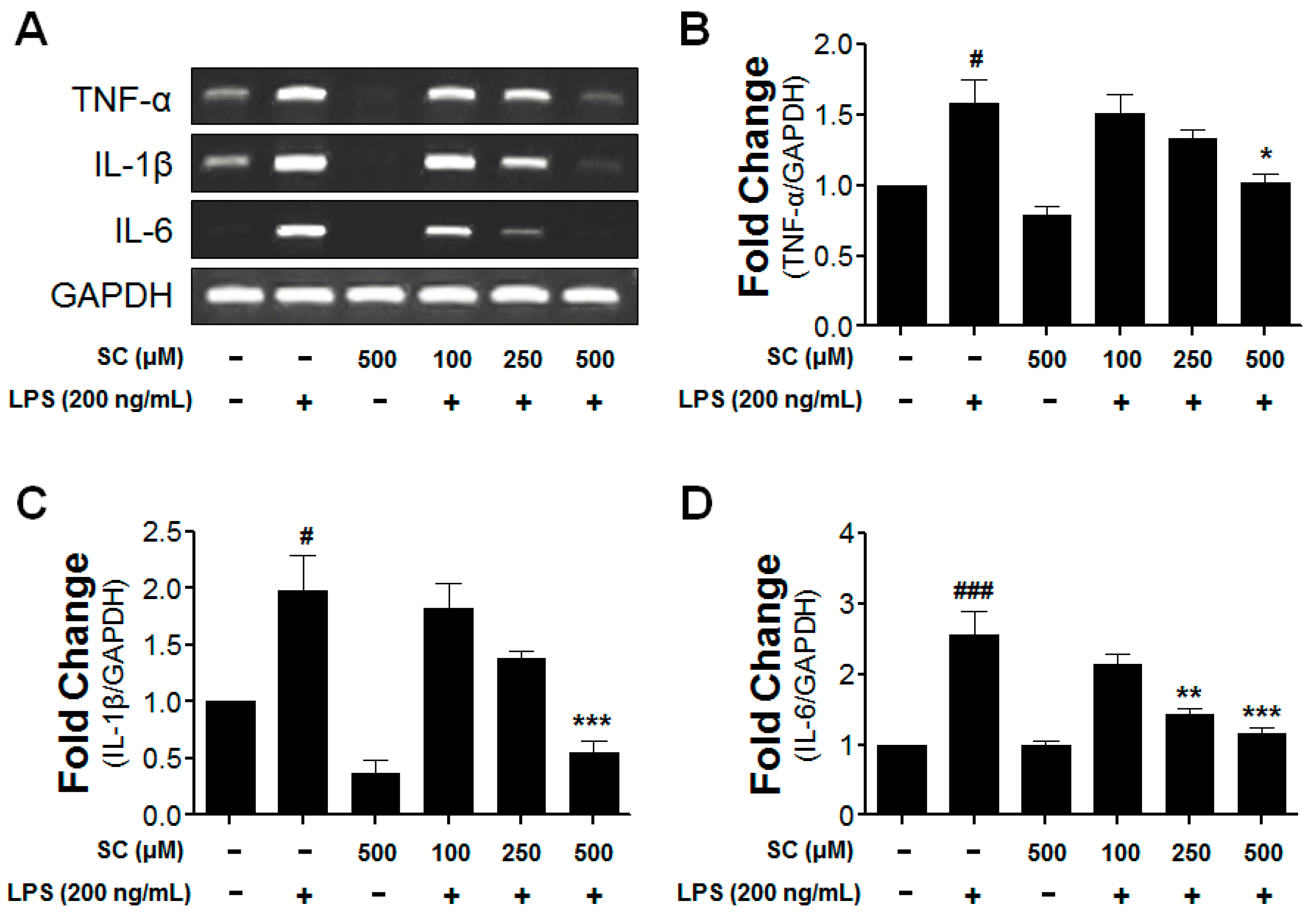
2.4. Inhibitory Effect of Scoparone on Proinflammatory-Cytokine Expression in BV-2 Microglial Cells
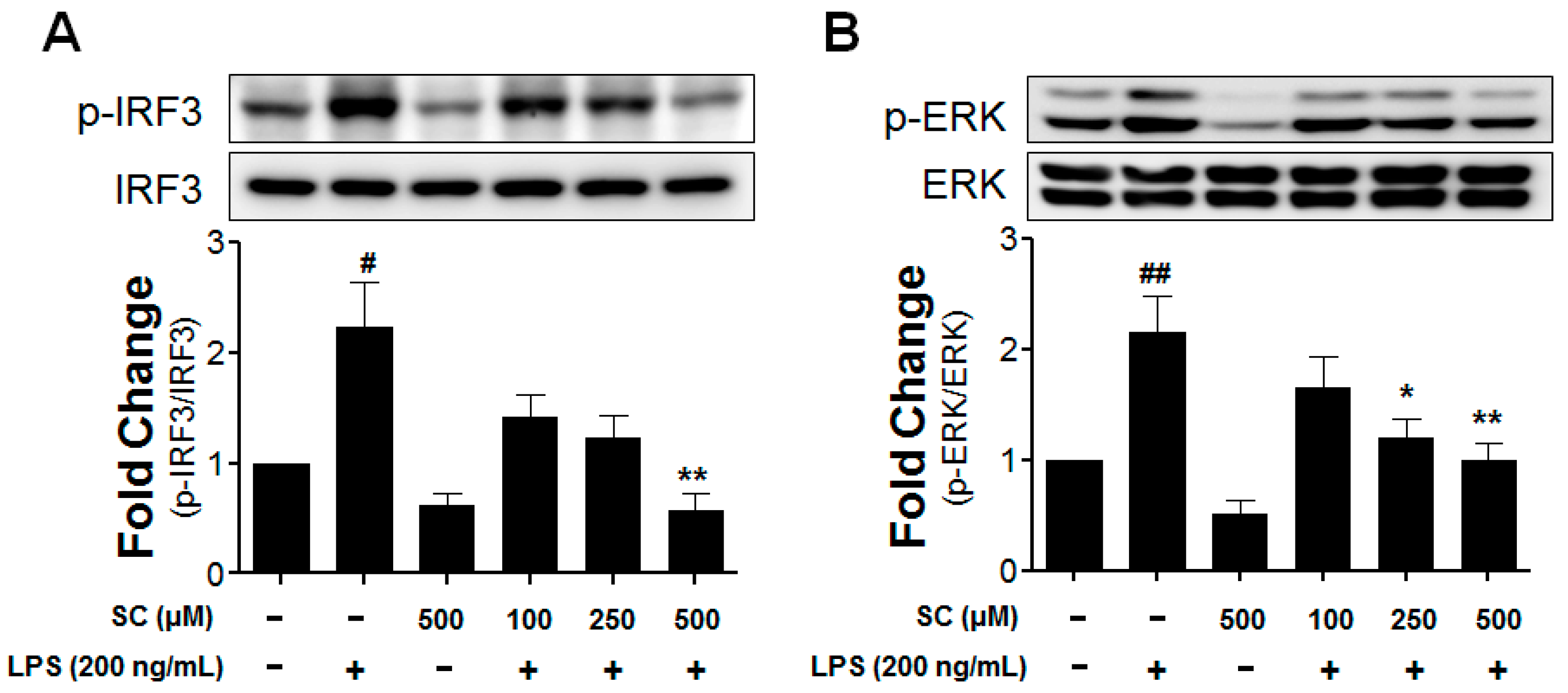
2.5. Effect of Scoparone on LPS-Induced Phosphorylation of IRF3 and ERK in BV-2 Microglial Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. BV-2 Microglial-Cell Culture
4.3. Cell Viability and Nitrite Assay
4.4. Total RNA Isolation and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Graeber, M.B.; Li, W.; Rodriguez, M.L. Role of microglia in CNS inflammation. FEBS Lett. 2011, 585, 3798–3805. [Google Scholar] [CrossRef] [PubMed]
- Von Bernhardi, R.; Tichauer, J.E.; Eugenin, J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J. Neurochem. 2010, 112, 1099–1114. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Han, S.J.; Yang, I.; Crane, C. Microglia and central nervous system immunity. Neurosurg. Clin. N. Am. 2010, 21, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Koppula, S.; Kumar, H.; Park, J.Y.; Kim, I.W.; More, S.V.; Kim, I.S.; Han, S.D.; Kim, S.K.; Yoon, S.H.; et al. Alpha-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology 2015, 97, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Park, J.I.; More, S.V.; Park, J.Y.; Kim, B.W.; Jeon, S.B.; Yun, Y.S.; Park, E.J.; Yoon, S.H.; Choi, D.K. Anti-neuroinflammatory effects of DPTP, a novel synthetic clovamide derivative in in vitro and in vivo model of neuroinflammation. Brain Res. Bull. 2015, 112, 25–34. [Google Scholar] [CrossRef] [PubMed]
- More, S.V.; Choi, D.K. Promising cannabinoid-based therapies for Parkinson’s disease: Motor symptoms to neuroprotection. Mol. Neurodegener. 2015, 10, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Rowe, D.C.; Barnes, B.J.; Caffrey, D.R.; Visintin, A.; Latz, E.; Monks, B.; Pitha, P.M.; Golenbock, D.T. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J. Exp. Med. 2003, 198, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.Y.; Lee, H.; Jung, B.Y.; Ock, J.; Lee, M.S.; Lee, W.H.; Suk, K. TLR4, but not TLR2, signals autoregulatory apoptosis of cultured microglia: A critical role of IFN-beta as a decision maker. J. Immunol. 2005, 174, 6467–6476. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Chai, J.C.; Kim, S.H.; Lee, Y.S.; Park, K.S.; Jung, K.H.; Chai, Y.G. Transcriptome sequencing of microglial cells stimulated with TLR3 and TLR4 ligands. BMC Genom. 2015, 16, 517. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.S.; Heo, M.Y.; Chung, J.H.; Jo, B.K.; Kim, H.P. Effects of the chestnut inner shell extract on the expression of adhesion molecules, fibronectin and vitronectin, of skin fibroblasts in culture. Arch. Pharm. Res. 2002, 25, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.R.; Gang, G.T.; Kim, Y.H.; Yang, K.J.; Hwang, J.H.; Lee, H.S.; Oh, W.K.; Song, K.S.; Lee, C.H. Antioxidant effects of the chestnut (Castanea crenata) inner shell extract in t-BHP-treated HepG2 cells, and CCl4- and high-fat diet-treated mice. Food Chem. Toxicol. 2010, 48, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, J.Y.; Kim, H.J.; Park, K.G.; Harris, R.A.; Cho, W.J.; Lee, J.T.; Lee, I.K. Scoparone exerts anti-tumor activity against DU145 prostate cancer cells via inhibition of STAT3 activity. PLoS ONE 2013, 8, e80391. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.K.; Oh, C.J.; Choi, S.H.; Jeon, J.H.; Lee, I.K. Scoparone interferes with STAT3-induced proliferation of vascular smooth muscle cells. Exp. Mol. Med. 2015, 47, e145. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Weng, Y.I.; Lee, C.R.; Jan, T.R.; Chen, Y.L.; Lee, Y.T. Protection by scoparone against the alterations of plasma lipoproteins, vascular morphology and vascular reactivity in hyperlipidaemic diabetic rabbit. Br. J. Pharmacol. 1993, 110, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Lee, H.J.; Choi, D.H.; Huang, H.S.; Lim, S.C.; Lee, M.K. Effect of scoparone on neurite outgrowth in PC12 cells. Neurosci. Lett. 2008, 440, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Lee, H.J.; Huang, H.S.; Lee, B.K.; Choi, H.S.; Lim, S.C.; Lee, C.K.; Lee, M.K. Effects of scoparone on dopamine biosynthesis and L-DOPA-induced cytotoxicity in PC12 cells. J. Neurosci. Res. 2009, 87, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Li, B.; Hu, Y.; Li, X.; Li, J.; Zhang, H. Protective effects of scoparone against lipopolysaccharide-induced acute lung injury. Int. Immunopharmacol. 2014, 23, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Hsiao, G.; Chang, J.W.; Sheu, J.R.; Yen, M.H. Scoparone inhibits tissue factor expression in lipopolysaccharide-activated human umbilical vein endothelial cells. J. Biomed. Sci. 2003, 10, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.M.; Kim, S.Y.; Joo, S.H.; Cheong, J.H.; Yang, S.I.; Shin, C.Y.; Koo, B.N. Synergistic activation of lipopolysaccharide-stimulated glial cells by propofol. Biochem. Biophys. Res. Commun. 2013, 438, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S. Innate immunity and neuroinflammation in the CNS: The role of microglia in Toll-like receptor-mediated neuronal injury. Glia 2010, 58, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, M.; Koistinaho, J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia 2002, 40, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Rodriguez-Perez, A.I.; Villar-Cheda, B.; Borrajo, A.; Dominguez-Meijide, A.; Guerra, M.J. Rho Kinase and Dopaminergic Degeneration: A Promising Therapeutic Target for Parkinson’s Disease. Neuroscientist 2015, 21, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Ko, H.M.; Kim, I.S.; Choi, D.K. Recent trends in the development of nanophytobioactive compounds and delivery systems for their possible role in reducing oxidative stress in Parkinson’s disease models. Int. J. Nanomed. 2015, 10, 6757–6772. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida, L.D., Jr.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Hoult, J.R.; Paya, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Salemme, A.; Togna, A.R.; Mastrofrancesco, A.; Cammisotto, V.; Ottaviani, M.; Bianco, A.; Venditti, A. Anti-inflammatory effects and antioxidant activity of dihydroasparagusic acid in lipopolysaccharide-activated microglial cells. Brain Res. Bull. 2016, 120, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hong, J.S. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Kim, Y.J.; Lee, W.Y.; Kwak, K.C.; Baek, S.H.; Kwak, G.B.; Yun, Y.G.; Kwon, T.O.; Chung, H.T.; Chai, K.Y. Scoparone from Artemisia capillaris inhibits the release of inflammatory mediators in RAW 264.7 cells upon stimulation cells by interferon-gamma Plus LPS. Arch. Pharm Res. 2005, 28, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Romieu-Mourez, R.; Solis, M.; Nardin, A.; Goubau, D.; Baron-Bodo, V.; Lin, R.; Massie, B.; Salcedo, M.; Hiscott, J. Distinct roles for IFN regulatory factor (IRF)-3 and IRF-7 in the activation of antitumor properties of human macrophages. Cancer Res. 2006, 66, 10576–10585. [Google Scholar] [CrossRef] [PubMed]
- Petrasek, J.; Dolganiuc, A.; Csak, T.; Nath, B.; Hritz, I.; Kodys, K.; Catalano, D.; Kurt-Jones, E.; Mandrekar, P.; Szabo, G. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology 2011, 53, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Wu, Y.; Li, L.Y.; Zheng, J.; Liu, R.G.; Zhou, J.P.; Yuan, S.Y.; Shang, Y.; Yao, S.L. Aspirin-triggered lipoxin A4 attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-kappaB and MAPKs in BV-2 microglial cells. J. Neuroinflamm. 2011, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.N.; Zong, Y.; Zhong, L.M.; Li, Y.M.; Zhang, W.; Bian, L.G.; Ai, Q.L.; Liu, Y.D.; Sun, J.; Lu, D. Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS ONE 2011, 6, e21891. [Google Scholar] [CrossRef] [PubMed]
- Rivest, S. Molecular insights on the cerebral innate immune system. Brain Behav. Immun. 2003, 17, 13–19. [Google Scholar] [CrossRef]
- Kim, B.W.; More, S.V.; Yun, Y.S.; Ko, H.M.; Kwak, J.H.; Lee, H.; Suk, K.; Kim, I.S.; Choi, D.K. A novel synthetic compound MCAP suppresses LPS-induced murine microglial activation in vitro via inhibiting NF-kB and p38 MAPK pathways. Acta Pharmacol. Sin. 2016, 37, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.M.; Koppula, S.; Kim, B.W.; Kim, I.S.; Hwang, B.Y.; Suk, K.; Park, E.J.; Choi, D.K. Inflexin attenuates proinflammatory responses and nuclear factor-kappaB activation in LPS-treated microglia. Eur. J. Pharmacol. 2010, 633, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds produced by chestnut inner shell (Castanea crenata) are available from the authors.



© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, D.-Y.; Ko, H.M.; Kim, J.; Kim, B.-W.; Yun, Y.-S.; Park, J.-I.; Ganesan, P.; Lee, J.-T.; Choi, D.-K. Scoparone Inhibits LPS-Simulated Inflammatory Response by Suppressing IRF3 and ERK in BV-2 Microglial Cells. Molecules 2016, 21, 1718. https://doi.org/10.3390/molecules21121718
Cho D-Y, Ko HM, Kim J, Kim B-W, Yun Y-S, Park J-I, Ganesan P, Lee J-T, Choi D-K. Scoparone Inhibits LPS-Simulated Inflammatory Response by Suppressing IRF3 and ERK in BV-2 Microglial Cells. Molecules. 2016; 21(12):1718. https://doi.org/10.3390/molecules21121718
Chicago/Turabian StyleCho, Duk-Yeon, Hyun Myung Ko, Joonsoo Kim, Byung-Wook Kim, Yo-Sep Yun, Jeong-In Park, Palanivel Ganesan, Jin-Tae Lee, and Dong-Kug Choi. 2016. "Scoparone Inhibits LPS-Simulated Inflammatory Response by Suppressing IRF3 and ERK in BV-2 Microglial Cells" Molecules 21, no. 12: 1718. https://doi.org/10.3390/molecules21121718
APA StyleCho, D. -Y., Ko, H. M., Kim, J., Kim, B. -W., Yun, Y. -S., Park, J. -I., Ganesan, P., Lee, J. -T., & Choi, D. -K. (2016). Scoparone Inhibits LPS-Simulated Inflammatory Response by Suppressing IRF3 and ERK in BV-2 Microglial Cells. Molecules, 21(12), 1718. https://doi.org/10.3390/molecules21121718






