Effects of Quercetin in a Rat Model of Hemorrhagic Traumatic Shock and Reperfusion
Abstract
:1. Introduction
2. Results
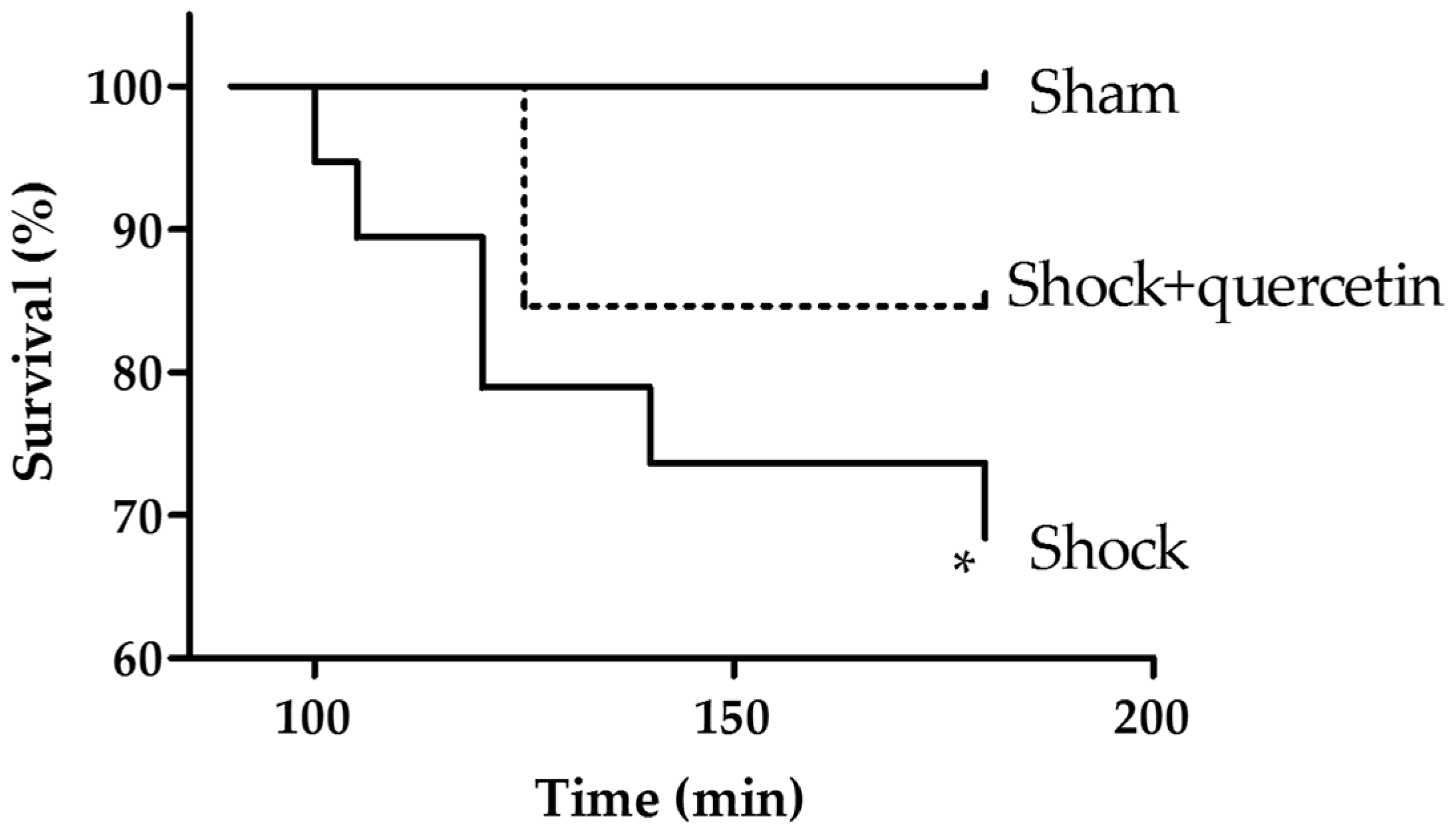
2.1. Mortality
2.2. Arterial Pressure and Heart Rate
2.3. Acid Sphingomyelinase Activity
2.4. Blood Gases
2.5. Electrolytes, Hematocrit and Hemoglobin
2.6. Markers of Inflammation, Pulmonary Vascular Permeability, Histological Changes and Bacterial Translocation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Anesthesia, Pressure Measurements and Blood Gases
4.3. Study Groups and Protocol
4.4. Myeloperoxidase Activity Assay (MPO)
4.5. Acid Sphingomyelinase Assay (aSMase)
4.6. Bacterial Translocation
4.7. Cytokine and IgM Assay
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Finfer, S.; Liu, B.; Taylor, C.; Bellomo, R.; Billot, L.; Cook, D.; Du, B.; McArther, C.; Myburgh, J. Resuscitation fluid use in critically ill adults: An international cross-sectional study in 391 intensive care units. Crit. Care 2010, 14, R185. [Google Scholar] [CrossRef] [PubMed]
- Collard, C.D.; Gelman, S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology 2001, 94, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Rushing, G.D.; Britt, L.D. Reperfusion injury after hemorrhage: A collective review. Ann. Surg. 2008, 247, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Cortés, I.; Peñuelas, O.; Esteban, A. Acute respiratory distress syndrome: Evaluation and management. Minerva Anestesiol. 2012, 78, 343–357. [Google Scholar] [PubMed]
- Pérez-Vizcaíno, F.; Ibarra, M.; Cogolludo, A.L.; Duarte, J.; Zaragozá-Arnáez, F.; Moreno, L.; López-López, G.; Tamargo, J. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J. Pharmacol. Exp. Ther. 2002, 302, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Duarte, J.; Jimenez, R.; Santos-Buelga, C.; Osuna, A. Antihypertensive effects of the flavonoid quercetin. Pharmacol. Rep. 2009, 61, 67–75. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Asp. Med. 2010, 31, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Xiao, W.; Zhang, H.; Lotze, M.T.; Wang, H.; Xiao, X. Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function. Am. J. Respir. Cell Mol. Biol. 2009, 41, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, R.; Barreira, B.; Moreno, E.; Lara-Acedo, V.; Morales-Cano, D.; Martínez-Ramas, A.; de Olaiz Navarro, B.; Herrero, R.; Lorente, J.A.; Cogolludo, A.; et al. Role of acid sphingomyelinase and IL-6 as mediators of endotoxin-induced pulmonary vascular dysfunction. Thorax 2016. [Google Scholar] [CrossRef] [PubMed]
- Von Bismarck, P. Improved Pulmonary Function by Acid Sphingomyelinase Inhibition in a Newborn Piglet Lavage Model. Am. J. Respir. Crit. Care Med. 2008, 177, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.H.; Barreto, J.C.; Klemm, K.; Miller, T.A. Hemorrhagic shock increases gut macromolecular permeability in the rat. Shock 1995, 4, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Harada, T.; Mollen, K.P.; Prince, J.M.; Levy, R.M.; Englert, J.A.; Gallowitsch-Puerta, M.; Yang, L.; Yang, H.; Tracey, K.J.; et al. AntiHMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol. Med. 2006, 12, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Enomoto, N.; Connor, H.D.; Moss, N.; Mason, R.P.; Thurman, R.G. Glycine improves survival after hemorrhagic shock in the rat. Shock 1999, 12, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.J.; Hinder, R.A.; Oosthuizen, M.M.J. Gastric mucosal injury caused by hemorrhagic shock and reperfusion: Protective role of the antioxidant glutathione. Surgery 1990, 108, 467–473. [Google Scholar] [PubMed]
- Hussmann, B.; Taeger, G.; Lefering, R.; Waydhas, C.; Nast-Kolb, D.; Ruchholtz, S.; Lendemans, S. Trauma register der Deutschen Gesell-schaft für Unfallchirurgie. Lethality and outcome in multiple injured patients after severe abdominal and pelvic trauma: Influence of preclinical volume replacement-an analysis of 604 patients from the trauma registry of thr DGU. Unfallchirung 2011, 114, 705–712. [Google Scholar]
- Rusznyak, S.; Szent-Györgyi, A. Vitamin P: Flavonols as vitamins. Nature 1936, 138, 27–29. [Google Scholar] [CrossRef]
- Gulbins, E.; Li, P.L. Physiological and pathophysiological aspects of ceramide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R11–R26. [Google Scholar] [CrossRef] [PubMed]
- Frazziano, G.; Moreno, L.; Moral-Sanz, J.; Menendez, C.; Escolano, L.; Gonzalez, C.; Villamor, E.; Alvarez-Sala, J.L.; Cogolludo, A.L.; Perez-Vizcaino, F. Neutral sphingomielinase, NADPH oxidase and reactive oxygen species. Role in acute hypoxic pulmonary vasoconstriction. J. Cell Physiol. 2011, 226, 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.J.; Tennagel, N. On ceramides, other sphingolipids and impaired glucose homeostasis. Mol. Metab. 2014, 3, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Tibboel, J.; Reiss, I.; de Jongste, J.C.; Post, M. Sphingolipids in Lung Growth and Repair. Chest 2014, 145, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Gulbins, E. Sphingolipids in the lungs. Am. J. Respir. Crit. Care Med. 2008, 178, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Göggel, R.; Winoto-Morbach, S.; Vielhaber, G.; Imai, Y.; Lindner, K.; Brade, L.; Brade, H.; Ehlers, S.; Slutsky, A.S.; Schütze, S.; et al. PAF-mediated pulmonary edema: A new role for acid sphingomyelinase and ceramide. Nat. Med. 2004, 10, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- De Oliveira, L.; Spiazzi, C.M.D.; Bortolin, T.; Canever, L.; Petronilho, F.; Mina, F.G.; Dal-Pizzol, F.; Quevedo, J.; Zugno, A.I. Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 2009, 33, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Nabavi, S.F.; Eslami, S.; Moghaddam, A.H. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012, 132, 931–935. [Google Scholar] [CrossRef]
- Doucet, D. Estrogen receptor hormone agonists limit trauma hemorrhage shock-induced gut and lung injury in rats. PLoS ONE 2010, 5, e9421. [Google Scholar] [CrossRef] [PubMed]
- Rönn, T. A new model of severe hemorrhagic shock in rats. Comp. Med. 2011, 61, 419–426. [Google Scholar] [PubMed]
- Mangala, A.; Nadkarni, A. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 2002, 148, 257–266. [Google Scholar]
- Sample Availability: Available from the authors.





| Group | Shock Phase (t = 90 min) | Reperfusion (t = 180 min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na | K | Ca | Hb | Hematocrit | Na | K | Ca | Hb | Hematocrit | |
| Sham | 137.4 ± 0.82 | 5.30 ± 0.13 | 1.28 ± 0.05 | 13.38 ± 0.37 | 39.40 ± 1.07 | 137.0 ± 2.84 | 5.10 ± 0.16 | 1.19 ± 0.11 | 12.62 ± 0.33 | 37.11 ± 0.95 |
| Shock | 132.0 ** ± 0.85 | 6.33 ** ± 0.26 | 1.18 ± 0.12 | 9.50 ** ± 0.50 | 27.88 ** ± 1.47 | 131.79 ± 2.37 | 5.84 ± 0.17 | 0.95 ± 0.09 | 11.59 ± 0.37 | 34.07 ± 1.09 |
| Shock + quercetin | nd | nd | nd | nd | nd | 133.36 ± 1.52 | 5.40 ± 0.17 | 1.18 ± 0.12 | 12.93 ± 0.42 | 38.00 ± 1.22 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, V.; Pandolfi, R.; Moreno, L.; Barreira, B.; Martínez-Ramas, A.; Morales-Cano, D.; Ruiz-Cabello, J.; Lorente, J.A.; Duarte, J.; Cogolludo, Á.; et al. Effects of Quercetin in a Rat Model of Hemorrhagic Traumatic Shock and Reperfusion. Molecules 2016, 21, 1739. https://doi.org/10.3390/molecules21121739
Chamorro V, Pandolfi R, Moreno L, Barreira B, Martínez-Ramas A, Morales-Cano D, Ruiz-Cabello J, Lorente JA, Duarte J, Cogolludo Á, et al. Effects of Quercetin in a Rat Model of Hemorrhagic Traumatic Shock and Reperfusion. Molecules. 2016; 21(12):1739. https://doi.org/10.3390/molecules21121739
Chicago/Turabian StyleChamorro, Virginia, Rachele Pandolfi, Laura Moreno, Bianca Barreira, Andrea Martínez-Ramas, Daniel Morales-Cano, Jesús Ruiz-Cabello, José Angel Lorente, Juan Duarte, Ángel Cogolludo, and et al. 2016. "Effects of Quercetin in a Rat Model of Hemorrhagic Traumatic Shock and Reperfusion" Molecules 21, no. 12: 1739. https://doi.org/10.3390/molecules21121739
APA StyleChamorro, V., Pandolfi, R., Moreno, L., Barreira, B., Martínez-Ramas, A., Morales-Cano, D., Ruiz-Cabello, J., Lorente, J. A., Duarte, J., Cogolludo, Á., Alvarez-Sala, J. L., & Perez-Vizcaino, F. (2016). Effects of Quercetin in a Rat Model of Hemorrhagic Traumatic Shock and Reperfusion. Molecules, 21(12), 1739. https://doi.org/10.3390/molecules21121739







