Combined Use of S. pombe and L. thermotolerans in Winemaking. Beneficial Effects Determined Through the Study of Wines’ Analytical Characteristics
Abstract
:1. Introduction
2. Results and Discussion
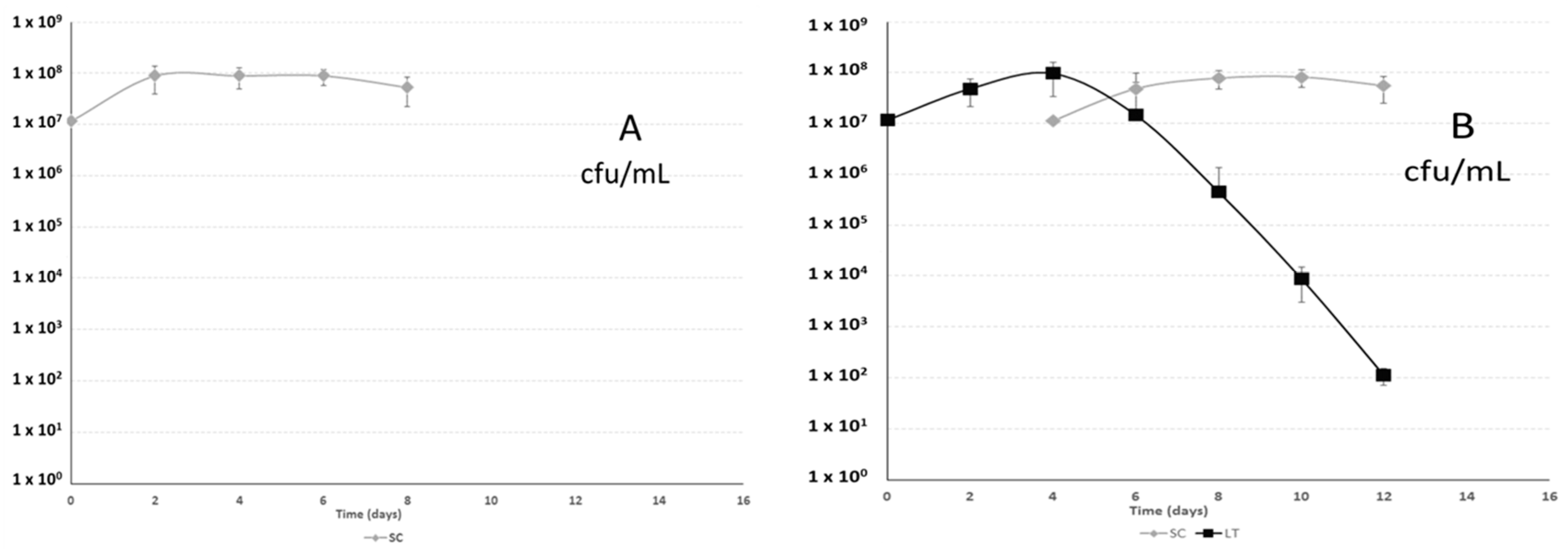
2.1. Yeast Population Kinetics
2.2. Sugar Consumption Kinetics
2.3. Acetic Acid
2.4. Malic Acid
2.5. L-Lactic Acid
2.6. Pyruvic Acid
2.7. Glycerol
2.8. Ethanol
2.9. Urea
2.10. Citric Acid
2.11. Volatile Aromatics
2.12. Biogenic Amines
2.13. Amino Acids
2.14. Colour Measurements
2.15. Ethanol Index
2.16. Sensory Evaluation
3. Materials and Methods
3.1. Microorganisms
3.2. Vinification
3.3. Measurements of Biochemical Compounds And pH
3.4. Microvinification Growth Kinetics
3.5. Quantification of Volatile Compounds
3.6. Quantification of Biogenic Amines
3.7. Analytical Determination of Amino Acids
3.8. Sensory Analysis
3.9. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and Future of Non-Saccharomyces Yeasts: From Spoilage Microorganisms to Biotechnological Tools for Improving Wine Aroma Complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Studies on Acetate Ester Production by Non-Saccharomyces Wine Yeasts. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of Non-Saccharomyces Wine Yeasts as Novel Sources of Mannoproteins in Wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Gong, H.S.; Jiang, X.M.; Zhao, Y.P. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces cerevisiae on Alcoholic Fermentation Behaviour and Wine Aroma of Cherry Wines. Food Microbiol. 2014, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Varela, C. The Impact of Non-Saccharomyces Yeasts in the Production of Alcoholic Beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic Analysis of Physiological Properties of Torulaspora delbrueckii in Wine Fermentations and its Incidence on Wine Quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Chua, J.; Huang, D.; Lee, P.; Liu, S. Biotransformation of Chemical Constituents of Durian Wine with Simultaneous Alcoholic Fermentation by Torulaspora delbrueckii. Appl. Microbiol. Biotechnol. 2016, 100, 8877–8888. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Reglero, G.; Herraiz, M.; Martin-Alvarez, P.J.; Cabezudo, M.D. The Influence of the Yeast and Type of Culture on the Volatile Composition of Wines Fermented without Sulfur Dioxide. Am. J. Enol. Vitic. 1990, 41, 313–318. [Google Scholar]
- Zironi, R.; Romano, P.; Suzzi, G.; Battistutta, F.; Comi, G. Volatile Metabolites Produced in Wine by Mixed and Sequential Cultures of Hanseniaspora guilliermondii or Kloeckera apiculata and Saccharomyces cerevisiae. Biotechnol. Lett. 1993, 15, 235–238. [Google Scholar] [CrossRef]
- Viana, F.; Belloch, C.; Vallés, S.; Manzanares, P. Monitoring a Mixed Starter of Hanseniaspora vineae–Saccharomyces cerevisiae in Natural must: Impact on 2-Phenylethyl Acetate Production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, S.; Genna, G.; Gandolfo, V.; Amore, G.; Ciaccio, M.; Oliva, D. Presence of Candida zemplinina in Sicilian Musts and Selection of a Strain for Wine Mixed Fermentations. S. Afr. J. Enol. Vitic. 2012, 33, 80–87. [Google Scholar]
- Belda, I.; Conchillo, L.B.; Ruiz, J.; Navascués, E.; Marquina, D.; Santos, A. Selection and use of Pectinolytic Yeasts for Improving Clarification and Phenolic Extraction in Winemaking. Int. J. Food Microbiol. 2016, 223, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Jeffares, D.; Palomero, F.; Calderón, F.; Bai, F.; Bähler, J.; Benito, S. Selected Schizosaccharomyces pombe Strains have Characteristics that are Beneficial for Winemaking. PloS ONE 2016, 11, e0151102. [Google Scholar] [CrossRef] [PubMed]
- Cañas, P.M.I.; García-Romero, E.; Manso, J.M.H.; Fernández-González, M. Influence of Sequential Inoculation of Wickerhamomyces anomalus and Saccharomyces cerevisiae in the Quality of Red Wines. European Food Res. Technol. 2014, 239, 279–286. [Google Scholar] [CrossRef]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on Cv. Emir Wine Fermentation. Yeast 2016, 33, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Benito, A.; Calderón, F.; Palomero, F.; Benito, S. Quality and Composition of Airen Wines Fermented by Sequential Inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.B.; Thornton, R.J. Factors Influencing the Utilisation of L-Malate by Yeasts. FEMS Microbiol. Lett. 1990, 72, 17–22. [Google Scholar]
- Thornton, R.; Rodriguez, S. Deacidification of Red and White Wines by a Mutant of Schizosaccharomyces malidevorans under Commercial Winemaking Conditions. Food Microbiol. 1996, 13, 475–482. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, P.; Calderón, F.; Palmero, D.; Suárez-Lepe, J.A. Schizosaccharomyces. In Encyclopedia of Food Microbiology, 2nd ed.; Batt CA, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 365–370. [Google Scholar]
- Palomero, F.; Morata, A.; Benito, S.; Calderón, F.; Suárez-Lepe, J. New Genera of Yeasts for Over-Lees Aging of Red Wine. Food Chem. 2009, 112, 432–441. [Google Scholar] [CrossRef]
- Romani, C.; Domizio, P.; Lencioni, L.; Gobbi, M.; Comitini, F.; Ciani, M.; Mannazzu, I. Polysaccharides and Glycerol Production by Non-Saccharomyces Wine Yeasts in Mixed Fermentation. Quad. Vitic. Enol. Univ. Torino. 2010, 31, 185–189. [Google Scholar]
- Peinado, R.A.; Mauricio, J.C.; Medina, M.; Moreno, J.J. Effect of Schizosaccharomyces pombe on Aromatic Compounds in Dry Sherry Wines Containing High Levels of Gluconic Acid. J. Agric. Food Chem. 2004, 52, 4529–4534. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.J.; Maestre, O.; Ortega, J.M.; Medina, M.; Mauricio, J.C. Gluconic Acid Consumption in Wines by Schizosaccharomyces pombe and its Effect on the Concentrations of Major Volatile Compounds and Polyols. J. Agric. Food Chem. 2004, 52, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.J.; Medina, M.; Mauricio, J.C. Potential Application of a Glucose-Transport-Deficient Mutant of Schizosaccharomyces pombe for Removing Gluconic Acid from Grape Must. J. Agric. Food Chem. 2005, 53, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.J.; Maestre, O.; Mauricio, J.C. Removing Gluconic Acid by using Different Treatments with a Schizosaccharomyces pombe Mutant: Effect on Fermentation Byproducts. Food Chem. 2007, 104, 457–465. [Google Scholar] [CrossRef]
- Peinado, R.A.; Maestre, O.; Mauricio, J.C.; Moreno, J.J. Use of a Schizosaccharomyces pombe Mutant to Reduce the Content in Gluconic Acid of must obtained from Rotten Grapes. J. Agric. Food Chem. 2009, 57, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Palomero, F.; Gálvez, L.; Morata, A.; Calderón, F.; Palmero, D.; Suárez-Lepe, J.A. Quality and Composition of Red Wine Fermented with Schizosaccharomyces pombe as Sole Fermentative Yeast, and in Mixed and Sequential Fermentations with Saccharomyces Cerevisiae. Food Technol. Biotechnol. 2014, 52, 376–382. [Google Scholar]
- Mylona, A.; Del Fresno, J.; Palomero, F.; Loira, I.; Bañuelos, M.; Morata, A.; Calderón, F.; Benito, S.; Suárez-Lepe, J. Use of Schizosaccharomyces Strains for Wine fermentation—Effect on the Wine Composition and Food Safety. Int. J. Food Microbiol. 2016, 232, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Suárez-Lepe, J.A. New Applications for Schizosaccharomyces pombe in the Alcoholic Fermentation of Red Wines. Int. J. Food Sci. Tech. 2012, 47, 2101–2108. [Google Scholar] [CrossRef]
- Benito, S. The use of Schizosaccharomyces Yeast in Order to Reduce the Content of Biogenic Amines and Ethyl Carbamate in Wines. J. Food Process Technol. 2015, 6, 9–10. [Google Scholar]
- Lubbers, M.W.; Rodriguez, S.B.; Honey, N.K.; Thornton, R.J. Purification and Characterization of Urease from Schizosaccharomyces pombe. Can. J. Microbiol. 1996, 42, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine use of Selected Schizosaccharomyces pombe and Lachancea thermotolerans Yeast Strains as an Alternative to the Traditional Malolactic Fermentation in Red Wine Production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [PubMed]
- Kapsopoulou, K.; Kapaklis, A.; Spyropoulos, H. Growth and Fermentation Characteristics of a Strain of the Wine Yeast Kluyveromyces thermotolerans Isolated in Greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological Acidification during Grape must Fermentation using Mixed Cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in Simultaneous and Sequential Co-Fermentation: A Strategy to Enhance Acidity and Improve the overall Quality of Wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, T.; Wang, Y.; Li, Y.; Li, H. The use of Lactic Acid-Producing, Malic Acid-Producing, Or Malic Acid-Degrading Yeast Strains for Acidity Adjustment in the Wine Industry. Appl. Microbiol. Biotechnol. 2014, 98, 2395–2413. [Google Scholar] [CrossRef] [PubMed]
- Snow, P.G.; Gallander, J.F. Deacidification of White Table Wines through Partial Fermentation with Schizosaccharomyces pombe. Am. J. Enol. Vitic. 1979, 30, 45–48. [Google Scholar]
- Unterholzner, O.; Aurich, M.; Platter, K. Geschmacks Und Geruchsfehler Bei Rotweinen Verursacht Durch Schizosaccharomyces pombe L. Mitteilungen Klosterneuburg, Rebe Und Wein. Obstbau und Früchteverwertung 1988, 38, 66–70. [Google Scholar]
- Yokotsuka, K.; Otaki, A.; Naitoh, A.; Tanaka, H. Controlled Simultaneous Deacidification and Alcohol Fermentation of a High-Acid Grape must using Two Immobilized Yeasts, Schizosaccharomyces pombe and Saccharomyces cerevisiae. Am. J. Enol. Vitic. 1993, 44, 371–377. [Google Scholar]
- Pitt, J.I.; Ailsa, D. Yeasts. In Fungi and Food Spoilage, 1st ed.; Pitt, J.I., Hocking, A.D., Eds.; Springer: Berlin, Germany, 2009; pp. 357–382. [Google Scholar]
- Benito, S.; Palomero, F.; Calderón, F.; Palmero, D.; Suárez-Lépe, J. Selection of Appropriate Schizosaccharomyces Strains for Winemaking. Food Microbiol. 2014, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Gálvez, L.; Palomero, F.; Morata, A.; Palmero, D.; Suarez-Lepe, J.A. Schizosaccharomyces Selective Differential Media. Afr. J. Microbiol. Res. 2013, 7, 3026–3036. [Google Scholar]
- Jeffares, D.C.; Rallis, C.; Rieux, A.; Speed, D.; Převorovský, M.; Mourier, T.; Marsellach, F.X.; Iqbal, Z.; Lau, W.; Cheng, T.M. The Genomic and Phenotypic Diversity of Schizosaccharomyces Pombe. Nat. Genet. 2015, 47, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Jolly, J.; Augustyn, O.; Pretorius, I. The Role and use of Non-Saccharomyces Yeasts in Wine Production. South Afr. J. Enol. Viticult. 2006, 27, 15–39. [Google Scholar]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on Quality and Composition of Riesling Wines Fermented by Sequential Inoculation with Non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Benito, S.; Morata, A.; Palomero, F.; Gonzalez, M.; Suárez-Lepe, J. Formation of Vinylphenolic Pyranoanthocyanins by Saccharomyces cerevisiae and Pichia guillermondii in Red Wines Produced Following Different Fermentation Strategies. Food Chem. 2011, 124, 15–23. [Google Scholar] [CrossRef]
- Bonciani, T.; Solieri, L.; de Vero, L.; Giudici, P. Improved Wine Yeasts by Direct Mating and Selection under Stressful Fermentative Conditions. Eur. Food Res. Technol. 2016, 242, 899–910. [Google Scholar] [CrossRef]
- García, M.; Greetham, D.; Wimalasena, T.T.; Phister, T.G.; Cabellos, J.M.; Arroyo, T. The Phenotypic Characterization of Yeast Strains to Stresses Inherent to Wine Fermentation in Warm Climates. J. Appl. Microbiol. 2016, 121, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Kutyna, D.R.; Varela, C.; Henschke, P.A.; Chambers, P.J.; Stanley, G.A. Microbiological Approaches to Lowering Ethanol Concentration in Wine. Trends Food Sci. Technol. 2010, 21, 293–302. [Google Scholar] [CrossRef]
- Gobbi, M.; de Vero, L.; Solieri, L.; Comitini, F.; Oro, L.; Giudici, P.; Ciani, M. Fermentative Aptitude of Non-Saccharomyces Wine Yeast for Reduction in the Ethanol Content in Wine. Eur. Food Res. Technol. 2014, 239, 41–48. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, F.; Morata, A.; Calderon, F.; Palmero, D.; Suarez-Lepe, J. Physiological Features of Schizosaccharomyces pombe of Interest in Making of White Wines. Eur. Food Res. Technol. 2013, 236, 29–36. [Google Scholar] [CrossRef]
- Röcker, J.; Strub, S.; Ebert, K.; Grossmann, M. Usage of Different Aerobic Non-Saccharomyces Yeasts and Experimental Conditions as a Tool for Reducing the Potential Ethanol Content in Wines. Eur. Food Res. Technol. 2016, 242, 2051–2070. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of Non-Saccharomyces Yeasts for the Reduction of Alcohol Content in Wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non-Conventional Yeast Species for Lowering Ethanol Content of Wines. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Curtin, C.; Varela, C. Yeast Population Dynamics Reveal a Potential ‘collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the Production of Reduced Alcohol Wines during Shiraz Fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The Impact of Oxygen on the Final Alcohol Content of Wine Fermented by a Mixed Starter Culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The use of Glucose Oxidase and Catalase for the Enzymatic Reduction of the Potential Ethanol Content in Wine. Food Chem. 2016, 210, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Deák, T. Yeasts. In Handbook of Food Spoilage, 2nd ed.; Deák, T., Ed.; CRC press: New York, NY, USA, 2007; pp. 294–296. [Google Scholar]
- Xia, C.; Hong, M.; YunFeng, Z.; YongNing, W. Research Progress on Toxicity and Contamination of Ethyl Carbamate in Fermented Foods. J. Food Saf. Qual. 2014, 5, 2617–2622. [Google Scholar]
- Shimazu, Y.; Uehara, M.; Watanabe, M. Transformation of Citric Acid to Acetic Acid, Acetoin and Diacetyl by Wine Making Lactic Acid Bacteria. Agric. Biol. Chem. 1985, 49, 2147–2157. [Google Scholar] [CrossRef]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Molecular Characterization and Oenological Properties of Wine Yeasts Isolated during Spontaneous Fermentation of Six Varieties of Grape Must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Parapouli, M.; Hatziloukas, E.; Drainas, C.; Perisynakis, A. The Effect of Debina Grapevine Indigenous Yeast Strains of Metschnikowia and Saccharomyces on Wine Flavour. J. Ind. Microbiol. Biotechnol. 2010, 37, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.; Suzzi, G.; Comi, G.; Zironi, R. Higher Alcohol and Acetic Acid Production by Apiculate Wine Yeasts. J. Appl. Bacteriol. 1992, 73, 126–130. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G. Higher Alcohol and Acetoin Production by Zygosaccharomyces Wine Yeasts. J. Appl. Bacteriol. 1993, 75, 541–545. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.; G-Alegría, E.; Polo, M.; Tenorio, C.; Martín-Álvarez, P.; Calvo De La Banda, M.T.; Ruiz-Larrea, F.; Moreno-Arribas, M. Wine Volatile and Amino Acid Composition After Malolactic Fermentation: Effect of Oenococcus oeni and Lactobacillus plantarum Starter Cultures. J. Agric. Food Chem. 2005, 53, 8729–8735. [Google Scholar] [CrossRef] [PubMed]
- Maynard, L.S.; Schenker, V.J. Monoamine Oxidase Inhibition by Ethanol in Vitro. Nature 1962, 575–576. [Google Scholar] [CrossRef]
- Kanny, G.; Gerbaux, V.; Olszewski, A.; Frémont, S.; Empereur, F.; Nabet, F.; Cabanis, J.; Moneret-Vautrin, D. No Correlation between Wine Intolerance and Histamine Content of Wine. J. Allergy Clin. Immunol. 2001, 107, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.C.; van Dusseldorp, M.; Bottema, K.C.; Dubois, A.E. Intolerance to Dietary Biogenic Amines: A Review. Ann. Allergy Asthma Immunol. 2003, 91, 233–241. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Polo, M.C. Occurrence of Lactic Acid Bacteria and Biogenic Amines in Biologically Aged Wines. Food Microbiol. 2008, 25, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, P. Determination of Amines and Amino Acids in Wine—A Review. Am. J. Enol. Vitic. 1996, 47, 127–133. [Google Scholar]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased Flavour Diversity of Chardonnay Wines by Spontaneous Fermentation and Co-Fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Hidalgo, J.M.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Polo, M.C. Influence of Malolactic Fermentation, Postfermentative Treatments and Ageing with Lees on Nitrogen Compounds of Red Wines. Food Chem. 2007, 103, 572–581. [Google Scholar] [CrossRef]
- Smit, A.Y.; du Toit, M. Evaluating the Influence of Malolactic Fermentation Inoculation Practices and Ageing on Lees on Biogenic Amine Production in Wine. Food Bioprocess Technol. 2013, 6, 198–206. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Implications of New Research and Technologies for Malolactic Fermentation in Wine. Appl. Microbiol. Biotechnol. 2014, 98, 8111–8132. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderón, F.; Benito, S. Outlining the Influence of Non-conventional Yeasts in Wine Ageing Over Lees. Yeast 2016, 33, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; Liu, Y.; Bisson, L.; Barile, D. Cell Wall Polysaccharides Released during the Alcoholic Fermentation by Schizosaccharomyces pombe and S. Japonicus: Quantification and Characterization. Food Microbiol. 2017, 61, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D.; Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. The chemistry of wine stabilization and treatments. In Handbook of Enology, 2nd ed.; Ribereau-Gayon, P., Ed.; Wiley: West Sussex, UK, 2006; pp. 141–203. [Google Scholar]
- Morris, E.; Eddy, A. Method for the Measurement of Wild Yeast Infection in Pitching Yeast. J. Inst. Brewing 1957, 63, 34–35. [Google Scholar] [CrossRef]
- Vaughnan-Martini, A.; Martini, A. Determination of Ethanol Production. In The Yeast. A Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 1999; p. 107. [Google Scholar]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, V. Methods for isolation, phenotypic characterization and maintenance of yeasts. In The Yeasts, a Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 87–110. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.







| Compound/Property | SC | SC + MLF | LT···SC | LT···SC + MLF | LT···SK | SK |
|---|---|---|---|---|---|---|
| l-Lactic Acid (g/L) | 0.01 ± 0.01 a | 0.73 ± 0.06 b | 2.96 ± 0.12 c | 3.71 ± 0.18 d | 3.41 ± 0.23 d | 0.02 ± 0.02 a |
| l-Malic Acid (g/L) | 1.14 ± 0.03 b | 0.01 ± 0.01 a | 1.10 ± 0.05 b | 0.01 ± 0.01 a | 0.01 ± 0.01 a | 0.01 ± 0.01 a |
| Acetic Acid (g/L) | 0.38 ± 0.02 a | 0.49 ± 0.04 b | 0.35 ± 0.03 a | 0.43 ± 0.04 b | 0.35 ± 0.04 a | 0.39 ± 0.02 a |
| Glucose+Fructose (g/L) | 1.55 ± 0.21 b | 0.09 ± 0.03 a | 1.76 ± 0.32 b | 0.14 ± 0.05 a | 1.98 ± 0.43 b | 1.72 ± 0.24 b |
| Glycerol (g/L) | 8.88 ± 0.02 a | 8.92 ± 0.04 a | 9.13 ± 0.05 b | 9.11 ± 0.08 b | 9.38 ± 0.06 c | 9.66 ± 0.02 d |
| Urea (mg/L) | 2.62 ± 0.02 b | 5.18 ± 0.08 c | 2.58 ± 0.05 b | 5.23 ± 0.11 c | 0.14 ± 0.04 a | 0.12 ± 0.02 b |
| Citric Acid (g/L) | 0.18 ± 0.01 b | 0.02 ± 0.01 a | 0.17 ± 0.04 b | 0.04 ± 0.03 a | 0.18 ± 0.03 b | 0.19 ± 0.01 b |
| Alcohol (% v/v) | 14.7 ± 0.0 d | 14.7 ± 0.0 d | 14.5 ± 0.0 c | 14.5 ± 0.0 c | 14.2 ± 0.0 a | 14.3 ± 0.0 b |
| pH | 3.90 ± 0.02 c | 3.96 ± 0.03 d | 3.71 ± 0.04 a | 3.75 ± 0.03 a | 3.69 ± 0.04 a | 4.06 ± 0.02 d |
| Compounds (mg/L) | SC | SC + MLF | LT···SC | LT···SC + MLF | LT···SK | SK |
|---|---|---|---|---|---|---|
| Acetaldehyde | 21.56 ± 1.88 c | 2.58 ± 0.27 a | 17.83 ± 2.56 cb | 3.12 ± 0.58 a | 15.32 ± 2.02 b | 16.21 ± 1.74 b |
| Ethyl lactate | 2.88 ± 0.22 a | 22.52 ± 1.16 d | 16.42 ± 0.89 b | 29.63 ± 2.32 e | 19.78 ± 1.02 c | 3.38 ± 0.31 a |
| Ethyl acetate | 19.42 ± 2.15 a | 32.42 ± 2.83 b | 21.58 ± 3.01 a | 30.62 ± 3.88 b | 19.83 ± 2.74 a | 18.77 ± 2.32 a |
| Diacetyl | 2.22 ± 0.18 a | 13.46 ± 1.08 b | 2.41 ± 0.37 a | 11.64 ± 2.36 b | 2.35 ± 0.41 a | 2.16 ± 0.21 a |
| Isoamyl acetate | 3.73 ± 0.45 b | 3.46 ± 0.41 b | 3.87 ± 0.82 b | 3.59 ± 0.91 b | 2.93 ± 0.98 ab | 2.12 ± 0.26 a |
| 1-Propanol | 24.41 ± 2.75 c | 24.92 ± 2.93 c | 28.51 ± 3.82 c | 29.02 ± 4.13 c | 18.21 ± 1.99 b | 12.56 ± 1.84 a |
| Isobutanol | 17.02 ± 2.11 b | 16.82 ± 2.31 b | 24.16 ± 2.31 c | 23.98 ± 2.61 c | 21.16 ± 2.92 bc | 7.88 ± 1.61 a |
| 1-Butanol | 5.44 ± 0.46 b | 2.86 ± 0.58 a | 7.12 ± 1.23 b | 4.89 ± 1.56 ab | 5.11 ± 0.82 b | 3.21 ± 0.42 a |
| 2-Methyl-butanol | 46.21 ± 4.53 d | 53.49 ± 4.87 d | 42.17 ± 5.78 cd | 49.21 ± 6.12 d | 32.07 ± 6.82 c | 22.16 ± 1.93 a |
| 3-Methyl-butanol | 29.23 ± 2.54 c | 31.56 ± 2.77 c | 27.76 ± 2.88 c | 30.11 ± 3.15 c | 19.12 ± 2.63 b | 14.83 ± 1.52 a |
| Isobutyl acetate | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ethyl butyrate | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Hexanol | 2.17 ± 0.18 b | 1.86 ± 0.21 b | 2.21 ± 0.32 b | 2.02 ± 0.38 b | 1.89 ± 0.39 b | 1.21 ± 0.14 a |
| 2-Phenyl-ethanol | 30.12 ± 1.88 b | 32.42 ± 2.59 b | 27.23 ± 2.64 b | 28.84 ± 3.56 b | 25.23 ± 3.06 ab | 22.35 ± 2.23 a |
| 2-Phenyl ethyl acetate | 6.85 ± 0.36 b | 7.13 ± 0.48 b | 6.41 ± 0.51 b | 6.66 ± 0.62 b | 6.02 ± 0.63 b | 5.21 ± 0.22 a |
| Compounds | Must | SC | SC + MLF | LT···SC | LT···SC + MLF | LT···SK | SK |
|---|---|---|---|---|---|---|---|
| Histamine (mg/L) | 0.13 ± 0.01 a | 0.12 ± 0.03 a | 0.56 ± 0.06 b | 0.16 ± 0.05 a | 0.51 ± 0.08 b | 0.13 ± 0.06 a | 0.18 ± 0.04 a |
| Tiramine (mg/L) | 0.09 ± 0.01 a | 0.08 ± 0.02 a | 0.12 ± 0.05 a | 0.10 ± 0.06 a | 0.14 ± 0.09 a | 0.11 ± 0.06 a | 0.09 ± 0.02 a |
| Phenylethylamine (g/L) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Putrescine (g/L) | 0.18 ± 0.02 a | 0.21 ± 0.03 a | 0.25 ± 0.09 a | 0.19 ± 0.06 a | 0.22 ± 0.08 a | 0.18 ± 0.07 a | 0.16 ± 0.03 a |
| Cadaverine (g/L) | 0.27 ± 0.02 a | 0.31 ± 0.03 a | 0.35 ± 0.07 a | 0.29 ± 0.07 a | 0.32 ± 0.09 a | 0.27 ± 0.06 a | 0.25 ± 0.03 a |
| Compounds (mg/L) | SC | SC + MLF | LT…SC | LT…SC + MLF | LT…SK | SK |
|---|---|---|---|---|---|---|
| Aspartic acid | 10.08 ± 0.61 a | 11.56 ± 0.92 a | 9.42 ± 1.02 a | 10.64 ± 1.33 a | 11.62 ± 1.78 a | 13.76 ± 0.71 b |
| Asparagine | 14.23 ± 1.08 ab | 15.39 ± 1.32 b | 12.48 ± 1.33 a | 13.62 ± 1.64 ab | 16.55 ± 1.52 b | 23.42 ± 1.43 c |
| Serine | 2.57 ± 0.41 a | 3.71 ± 0.52 b | 2.47 ± 0.50 a | 3.62 ± 0.58 b | 3.88 ± 0.64 b | 5.11 ± 0.72 c |
| Histidine | 58.42 ± 2.79 b | 50.63 ± 3.08 a | 61.31 ± 3.92 b | 52.53 ± 4.21 a | 62.93 ± 4.32 b | 79.21 ± 3.22 c |
| Glycine | 25.22 ± 1.35 b | 29.86 ± 1.61 c | 16.42 ± 2.16 a | 19.76 ± 2.52 a | 16.96 ± 2.22 a | 26.14 ± 1.44 b |
| Arginine | 56.42 ± 2.79 b | 45.63 ± 3.08 a | 57.31 ± 3.92 b | 48.53 ± 3.21 a | 55.26 ± 3.43 b | 70.36 ± 4.79 c |
| Threonine | 19.36 ± 1.89 a | 21.26 ± 2.14 a | 18.24 ± 2.11 a | 20.13 ± 2.45 a | 28.42 ± 2.93 b | 46.58 ± 4.01 c |
| Alanine | 26.31 ± 2.41 a | 35.52 ± 2.96 a | 25.77 ± 2.88 a | 33.66 ± 3.17 a | 26.52 ± 2.72 a | 24.14 ± 1.99 a |
| Tyrosine | 3.75 ± 0.21 a | 3.89 ± 0.29 a | 3.67 ± 0.36 a | 3.93 ± 0.45 a | 4.12 ± 0.98 a | 7.38 ± 0.74 b |
| Valine | 1.36 ± 0.14 b | 1.35 ± 0.58 b | 0.32 ± 0.28 a | 0.33 ± 0.45 a | 1.32 ± 0.65 b | 5.89 ± 0.44 d |
| Tryptophan | 0.24 ± 0.04 a | 0.55 ± 0.16 b | 0.28 ± 0.07 a | 0.61 ± 0.21 b | 1.05 ± 0.76 bc | 2.11 ± 0.31 c |
| Phenylalanine | 2.96 ± 0.27 a | 3.17 ± 0.38 a | 2.77 ± 0.41 a | 3.02 ± 0.55 a | 4.67 ± 0.72 b | 6.82 ± 0.55 c |
| Isoleucine | 3.16 ± 0.23 b | 2.76 ± 0.31 a | 5.22 ± 0.41 d | 4.28 ± 0.67 c | 8.87 ± 0.87 e | 14.16 ± 0.82 f |
| Leucine | 3.84 ± 0.42 b | 2.53 ± 0.51 a | 4.92 ± 0.62 d | 4.11 ± 0.87 bd | 9.96 ± 1.12 e | 18.43 ± 1.06 f |
| Ornithine | 38.21 ± 2.17 c | 44.37 ± 2.88 d | 36.32 ± 3.21 c | 42.16 ± 3.94 cd | 28.33 ± 2.51 b | 21.15 ± 1.82 a |
| Lysine | 8.52 ± 0.77 a | 9.68 ± 0.86 a | 8.96 ± 1.35 a | 10.58 ± 1.33 a | 8.78 ± 1.72 a | 15.16 ± 1.02 b |
| Methionine | 1.11 ± 0.18 a | 1.82 ± 0.31 b | 1.08 ± 0.24 a | 1.68 ± 0.44 ab | 1.46 ± 0.31 ab | 2.45 ± 0.29 c |
| Compounds | SC | SC + MLF | LT…SC | LT…SC + MLF | LT…SK | SK |
|---|---|---|---|---|---|---|
| 420 nm | 6 ± 1 a | 6 ± 1 a | 7 ± 1 a | 7 ± 1 a | 7 ± 1 a | 08 ± 1 a |
| 520 nm | 8 ± 1 b | 6 ± 1 a | 9 ± 1 b | 7 ± 1 a | 9 ± 1 bc | 10 ± 1 c |
| 620 nm | 2 ± 1 a | 2 ± 1 a | 1 ± 1 a | 1 ± 1 a | 02 ± 1 a | 2 ± 1 a |
| Cia< | 16 ± 1 ab | 14 ± 1 a | 17 ± 1 ab | 15 ± 1 a | 18 ± 1 bc | 20 ± 1 c |
| Hue | 0.75 ± 0.02 a | 1.00 ± 0.02 b | 0.77 ± 2 a | 1.00 ± 0.02 b | 0.77 ± 0.02 a | 0.80 ± 0.02 a |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benito, Á.; Calderón, F.; Benito, S. Combined Use of S. pombe and L. thermotolerans in Winemaking. Beneficial Effects Determined Through the Study of Wines’ Analytical Characteristics. Molecules 2016, 21, 1744. https://doi.org/10.3390/molecules21121744
Benito Á, Calderón F, Benito S. Combined Use of S. pombe and L. thermotolerans in Winemaking. Beneficial Effects Determined Through the Study of Wines’ Analytical Characteristics. Molecules. 2016; 21(12):1744. https://doi.org/10.3390/molecules21121744
Chicago/Turabian StyleBenito, Ángel, Fernando Calderón, and Santiago Benito. 2016. "Combined Use of S. pombe and L. thermotolerans in Winemaking. Beneficial Effects Determined Through the Study of Wines’ Analytical Characteristics" Molecules 21, no. 12: 1744. https://doi.org/10.3390/molecules21121744






