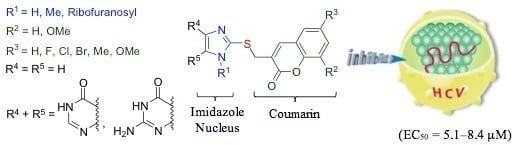
Synthesis and Structure-Activity Relationships of Imidazole-Coumarin Conjugates against Hepatitis C Virus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
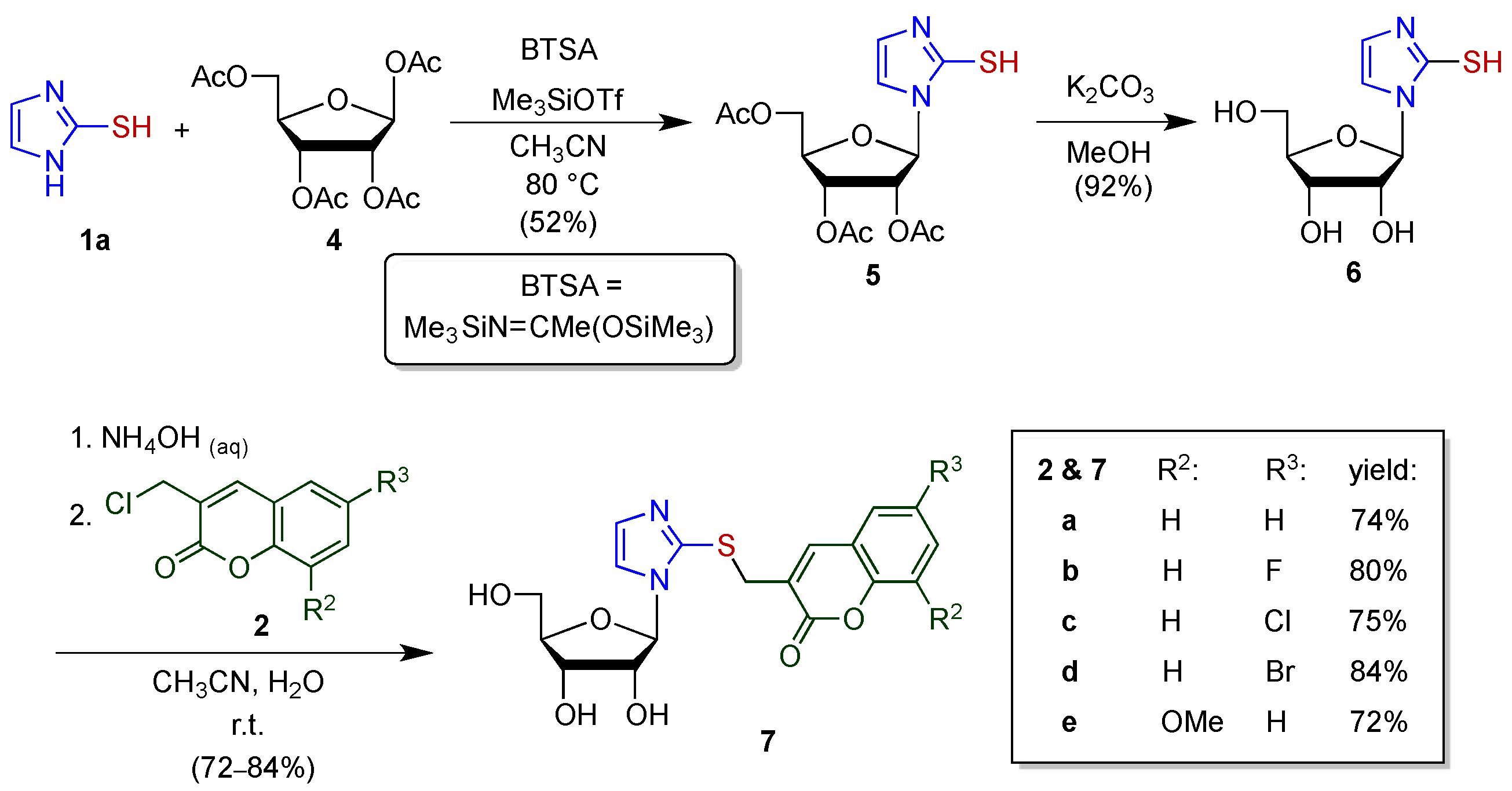
2.1.1. Synthesis of Imidazole–Coumarin Conjugates
2.1.2. Synthesis of (1-Ribofuranosyl)imidazole–Coumarin Conjugates
2.1.3. Synthesis of Inosine– and Guanosine–Coumarin Conjugates
2.1.4. Identification of the Structures of New Conjugated Compounds
2.2. Pharmacology
Anti-HCV Activity
3. Structure–Activity Relationship: Essential Moieties and Functional Groups
4. Experimental Section
4.1. General Procedures
4.2. Standard Procedure for the Synthesis of Imidazole–Coumarin Conjugates 3, 7, 9, and 11
4.2.1. 2-[(Coumarin-3’-yl)methylthio]imidazole (3a)
4.2.2. 2-[(6′-Fluorocoumarin-3′-yl)methylthio]imidazole (3b)
4.2.3. 2-[(6′-Chlorocoumarin-3′-yl)methylthio]imidazole (3c)
4.2.4. 2-[(6′-Bromocoumarin-3′-yl)methylthio]imidazole (3d)
4.2.5. 2-[(8′-Methoxycoumarin-3′-yl)methylthio]imidazole (3e)
4.2.6. 1-Methyl-2-[(coumarin-3′-yl)methylthio]imidazole (3f)
4.2.7. 1-Methyl-2-[(6′-chlorocoumarin-3′-yl)methylthio]imidazole (3g)
4.2.8. 1-(2′,3′,5′-Tri-O-acetyl-β-d-ribofuranos-1′-yl)imidazole-2-thiol (5)
4.2.9. 1-β-d-Ribofuranosyl-imidazole-2-thiol (6)
4.2.10. 1-(β-d-Ribofuranos-1″-yl)-2-[(coumarin-3′-yl)methylthio]imidazole (7a)
4.2.11. 1-(β-d-Ribofuranos-1″-yl)-2-[(6′-fluorocoumarin-3′-yl)methylthio]imidazole (7b)
4.2.12. 1-(β-d-Ribofuranos-1″-yl)-2-[(6′-chlorocoumarin-3′-yl)methylthio]imidazole (7c)
4.2.13. 1-(β-d-Ribofuranos-1″-yl)-2-[(6′-bromocoumarin-3′-yl)methylthio]imidazole (7d)
4.2.14. 1-(β-d-Ribofuranos-1″-yl)-2-[(8′-methoxycoumarin-3′-yl)methylthio]imidazole (7e)
4.2.15. 8-[(Coumarin-3′-yl)methylthio]inosine (9a)
4.2.16. 8-[(6′-Fluorocoumarin-3′-yl)methylthio]inosine (9b)
4.2.17. 8-[(6′-Bromocoumarin-3′-yl)methylthio]inosine (9d)
4.2.18. 8-[(8′-Methoxycoumarin-3′-yl)methylthio]inosine (9e)
4.2.19. 8-[(6′-Methylcoumarin-3′-yl)methylthio]inosine (9f)
4.2.20. 8-[(6′-Fluorocoumarin-3′-yl)methylthio]guanosine (11b)
4.2.21. 8-[(8′-Methoxycoumarin-3′-yl)methylthio]guanosine (11e)
4.2.22. 8-[(6′-Methylcoumarin-3′-yl)methylthio]guanosine (11f)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murphy, D.G.; Sablon, E.; Chamberland, J.; Fournier, E.; Dandavino, R.; Tremblay, C.L. Hepatitis C virus genotype 7, a new genotype originating from central Africa. J. Clin. Microbiol. 2015, 53, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Oze, T.; Hiramatsu, N.; Mita, E.; Akuta, N.; Sakamoto, N.; Nagano, H.; Itoh, Y.; Kaneko, S.; Izumi, N.; Nomura, H.; et al. A multicenter survey of re-treatment with pegylated interferon plus ribavirin combination therapy for patients with chronic hepatitis C in Japan. Hepatol. Res. 2013, 43, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.D.; Kauffman, R.S.; Hurter, P.; Mueller, P. Discovery and development of telaprevir: An NS3–4A protease inhibitor for treating genotype 1 chronic hepatitis C virus. Nat. Biotechnol. 2011, 29, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Thibault, P.A.; Wilson, J.A. Targeting miRNAs to treat hepatitis C virus infections and liver pathology: Inhibiting the virus and altering the host. Pharmacol. Res. 2013, 75, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Hulskotte, E.G.J.; Feng, H.-P.; Xuan, F.; Gupta, S.; van Zutven, M.G.J.A.; O’Mara, E.; Wagner, J.A.; Butterton, J.R. Pharmacokinetic evaluation of the interaction between hepatitis C virus protease inhibitor boceprevir and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and pravastatin. Antimicrob. Agents Chemother. 2013, 57, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Orrin, E.; Barnabas, A.; Agarwal, K.; Walsh, S.A. Cutaneous side-effects of antihepatitis C treatment: The U.K. experience. Br. J. Dermatol. 2015, 172, 292–293. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Approves First Combination Pill to Treat Hepatitis C. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm418365.htm (accessed on 10 October 2014).
- U.S. Food and Drug Administration. FDA Approves Viekira Pak to Treat Hepatitis C. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427530.htm (accessed on 19 December 2014).
- Van de Ven, N.; Fortunak, J.; Simmons, B.; Ford, N.; Cooke, G.S.; Khoo, S.; Hill, A. Minimum target prices for production of direct-acting antivirals and associated diagnostics to combat hepatitis C virus. Hepatology 2015, 61, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Tsay, S.-C.; Hwu, J.R.; Singha, R.; Huang, W.-C.; Chang, Y.H.; Hsu, M.-H.; Shieh, F.-K.; Lin, C.-C.; Hwang, K.C.; Horng, J.-C.; et al. Coumarins hinged directly on benzimidazoles and their ribofuranosides to inhibit hepatitis C virus. Eur. J. Med. Chem. 2013, 63, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Singha, R.; Hong, S.-C.; Chang, Y.-H.; Das, A.R.; Vliegen, I.; de Clercq, E.; Neyts, J. Synthesis of new benzimidazole–coumarin conjugates as anti-hepatitis C virus agents. Antivir. Res. 2008, 77, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Neyts, J.; de Clercq, E.; Singha, R.; Chang, Y.-H.; Das, A.R.; Chakraborty, S.K.; Hong, S.-C.; Tsay, S.-C.; Hsu, M.-H.; Hwu, J.R. Structure-activity relationship of new anti-hepatitis C virus agents: Heterobicycle-coumarin conjugates. J. Med. Chem. 2009, 52, 1486–1490. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Lin, S.-Y.; Tsay, S.-C.; de Clercq, E.; Leyssen, P.; Neyts, J. Coumarin-purine ribofuranoside conjugates as new agents against hepatitis C virus. J. Med. Chem. 2011, 54, 2114–2126. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Lin, S.-Y.; Tsay, S.-C.; Singha, R.; Pal, B.K.; Leyssen, P.; Neyts, J. Development of New Sulfur-Containing Conjugated Compounds as Anti-HCV Agents. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 1144–1152. [Google Scholar] [CrossRef]
- Nichols, D.B.; Leão, R.A.; Basu, A.; Chudayeu, M.; de Moraes, P.F.; Talele, T.T.; Costa, P.R.; Kaushik–Basu, N. Evaluation of coumarin and neoflavone derivatives as HCV NS5B polymerase inhibitors. Chem. Biol. Drug Des. 2013, 81, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Cervi, A.; Aillard, P.; Hazeri, N.; Petit, L.; Chai, C.L.L.; Willis, A.C.; Banwell, M.G. Total syntheses of the coumarin-containing natural products pimpinellin and fraxetin using Au(I)-catalyzed intramolecular hydroarylation (IMHA) chemistry. J. Org. Chem. 2013, 78, 9876–9882. [Google Scholar] [CrossRef] [PubMed]
- François, J.; Raymond, A. Small-Molecule Hepatitis C Virus (HCV) ns3/4a Serine Protease Inhibitors. WO Patent 128344 A1, 30 October 2008. [Google Scholar]
- Njoroge, F.G.; Chen, K.X.; Shih, N.-Y.; Piwinski, J.J. Challenges in modern drug discovery: A case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection. Acc. Chem. Res. 2008, 41, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Kwong, A.D.; Perni, R.B. Discovery and development of VX-950, a novel, covalent, and reversible inhibitor of hepatitis C virus NS3.4A serine protease. Infect. Disord. Drug Targets 2006, 6, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Khabnadideh, S.; Rezaei, Z.; Khalafi–Nezhad, A.; Bahrinajafi, R.; Mohamadi, R.; Farrokhroz, A.A. Synthesis of N-alkylated derivatives of imidazole as antibacterial agents. Bioorg. Med. Chem. Lett. 2003, 13, 2863–2865. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sharma, P.K.; Kumar, N. A review on “imidazoles”: Their chemistry and pharmacological potentials. Int. J. Pharmtech Res. 2011, 3, 268–282. [Google Scholar]
- Chen, J.; Wang, Z.; Li, C.M.; Lu, Y.; Vaddady, P.K.; Meibohm, B.; Dalton, J.T.; Miller, D.D.; Li, W. Discovery of novel 2-aryl-4-benzoyl-imidazoles targeting the colchicines binding site in tubulin as potential anticancer agents. J. Med. Chem. 2010, 53, 7414–7427. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Narasimhan, B.; Kumar, P.; Judge, V.; Narang, R.; de Clercq, E.; Balzarini, J. Synthesis, antimicrobial and antiviral evaluation of substituted imidazole derivatives. Eur. J. Med. Chem. 2009, 44, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Lagoja, I.M.; Pannecouque, C.; van Aerschot, A.; Witvrouw, M.; Debyser, Z.; Balzarini, J.; Herdewijn, P.; de Clercq, E. N-Aminoimidazole derivatives inhibiting retroviral replication via a yet unidentified mode of action. J. Med. Chem. 2003, 46, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.; Yi, M. The proton transfer properties of imidazole. J. Phys. Chem. 1996, 100, 9235–9241. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.-M.; Wang, J.; Ahn, S.; Wang, Z.; Lu, Y.; Dalton, J.T.; Miller, D.D.; Li, W. Synthesis and antiproliferative activity of novel 2-aryl-4-benzoyl-imidazole derivatives targeting tubulin polymerization. Bioorg. Med. Chem. 2011, 19, 4782–4795. [Google Scholar] [CrossRef] [PubMed]
- Fasciano, J.; Steele, T.; Castagnoli, N.; Katz, J.; Ricaurte, G. The effect of N-methylation on fenfluramine’s neurotoxic and pharmacologic actions. Brain Res. 1997, 763, 182–190. [Google Scholar] [CrossRef]
- Zai, L.; Ferrari, C.; Subbaiah, S.; Havton, L.A.; Coppola, G.; Strittmatter, S.; Irwin, N.; Geschwind, D.; Benowitz, L.I. Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J. Neurosci. 2009, 29, 8187–8197. [Google Scholar] [CrossRef] [PubMed]
- Liaudet, L.; Mabley, J.G.; Pacher, P.; Virag, L.; Soriano, F.G.; Marton, A.; Haskó, G.; Deitch, E.A.; Szabó, C. Inosine exerts a broad range of anti-inflammatory effects in a murine model of acute lung injury. Ann. Surg. 2002, 235, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Cianciara, J.; Laskus, T.; Gabinska, E.; Loch, T. Isoprinosine in the treatment of chronic active hepatitis type B. Scand. J. Infect. Dis. 1990, 22, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Kowalik-Mikołajewska, B. Course of chronic virus hepatitis B in children and attempts at modifying its treatment. Pol. Tyg. Lek. 1993, 48, 258–260. [Google Scholar] [PubMed]
- Njei, B.; Garg, S.K.; Kenta-Bibi, E.; Kongnyuy, E.J.; Alam, S.E. Isoprinosine for chronic hepatitis B. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Giuliani, P.; Romano, S.; Ballerini, P.; Ciccarelli, R.; Petragnani, N.; Cicchitti, S.; Zuccarini, M.; Jiang, S.; Rathbone, M.P.; Caciagli, F.; et al. Protective activity of guanosine in an in vitro model of Parkinson’s disease. Panminerva Med. 2012, 54, 43–51. [Google Scholar] [PubMed]
- Sizun, G.; Pierra, C.; Peyronnet, J.; Badaroux, E.; Rabeson, C.; Benzaria-Prad, S.; Surleraux, D.; Loi, A.G.; Musiu, C.; Liuzzi, M.; et al. Design, synthesis and antiviral evaluation of 2′-C-methyl branched guanosine pronucleotides: The discovery of IDX184, a potent liver-targeted HCV polymerase inhibitor. Future Med. Chem. 2015, 7, 1675–1700. [Google Scholar] [CrossRef] [PubMed]
- Ali-von Laue, C.; Zoschke, C.; Do, N.; Lehnen, D.; Küchler, S.; Mehnert, W.; Blaschke, T.; Kramer, K.D.; Plendl, J.; Weindl, G.; et al. Improving topical non-melanoma skin cancer treatment: In vitro efficacy of a novel guanosine-analog phosphonate. Skin Pharmacol. Physiol. 2014, 27, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.; Wu, Q.; Htar, T.T. Recent advances in antiviral nucleoside and nucleotide therapeutics. Curr. Top. Med. Chem. 2005, 5, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Chien, T.C.; Saluja, S.S.; Drach, J.C.; Townsend, L.B. Synthesis and antiviral evaluation of polyhalogenated imidazole nucleosides: Dimensional analogues of 2,5,6-trichloro-1-(β-d-ribofuranosyl)- benzimidazole. J. Med. Chem. 2004, 47, 5743–5752. [Google Scholar] [CrossRef] [PubMed]
- Bhan, P.; Bhan, A.; Hong, M.; Hartwell, J.G.; Saunders, J.M.; Hoke, G.D. 2′,5′-Linked oligo-3′-deoxyribonucleoside phosphorothioate chimeras: Thermal stability and antisense inhibition of gene expression. Nucleic Acids Res. 1997, 25, 3310–3317. [Google Scholar] [CrossRef] [PubMed]
- Chávez–Gil, T.E.; Meléndez, E. Synthesis, spectroscopic and electrochemical characterization of water soluble [(η5-C5H5)2Mo(thionucleobase/thionucleoside)]Cl2 complexes. Inorg. Chim. Acta 2004, 357, 1092–1102. [Google Scholar] [CrossRef]
- Lin, T.-S.; Cheng, J.-C.; Ishiguro, K.; Sartorelli, A.C. Purine and 8-substituted purine arabinofuranosyl and ribofuranosyl nucleoside derivatives as potential inducers of the differentiation of the Friend erythroleukemia. J. Med. Chem. 1985, 28, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-S.; Cheng, J.-C.; Ishiguro, K.; Sartorelli, A.C. 8-Substituted guanosine and 2′-deoxyguanosine derivatives as potential inducers of the differentiation of Friend erythroleukemia cells. J. Med. Chem. 1985, 28, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley: New York, NY, USA, 2005; pp. 92–94. [Google Scholar]
- Vorbrüggen, H.; Krolikiewicz, K.; Bennua, B. Nucleoside syntheses, XXII Nucleoside synthesis with trimethylsilyl triflate and perchlorate as catalysts. Chem. Ber. 1981, 114, 1234–1255. [Google Scholar] [CrossRef]
- Al Mourabit, A.; Beckmann, M.; Poupat, C.; Ahond, A.; Potier, P. New C2 symmetrical and semisymmetrical substituted imidazolium ribonucleoside. Imidazolic nucleosides analogues. Tetrahedron Asymmetry 1996, 7, 3455–3464. [Google Scholar] [CrossRef]
- Lohmann, V.; Körner, F.; Koch, J.-O.; Herian, U.; Theilmann, L.; Bartenschlager, R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 1999, 285, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Paeshuyse, J.; Kaul, A.; de Clercq, E.; Rosenwirth, B.; Dumont, J.-M.; Scalfaro, P.; Bartenschlager, R.; Neyts, J. The non-immunosuppressive cyclosporin DEBIO-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology 2006, 43, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Kaye, P.T.; Musa, M.A. A convenient and improved Baylis-Hillman synthesis of 3-substituted 2H-1-benzopyran-2-ones. Synthesis 2002, 2701–2706. [Google Scholar] [CrossRef]
- Gosselin, G.; Imbach, J.L.; Townsend, L.B.; Panzica, R.P. The synthesis of imidazole-2-thione nucleosides. J. Heterocycl. Chem. 1979, 16, 1185–1191. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all conjugated compounds are available from the authors.




| Compound a | CC50 b (μM) | EC50 c (μM) | SI d |
|---|---|---|---|
| 3a | 122 | 30 | 4.1 |
| 3b | 83 | 7.2 | 12 |
| 3c | 85 | 9.7 | 8.8 |
| 3d | 75 | 5.1 | 15 |
| 3e | 173 | 8.4 | 21 |
| 3f | 49 | 6.7 | 7.3 |
| 3g | 75 | 15 | 5.1 |
| 7a | 128 | 59 | 2.2 |
| 7b | 122 | 26 | 4.7 |
| 7c | 85 | 14 | 6.3 |
| 7d | 107 | 19 | 5.7 |
| 7e | 119 | 34 | 3.5 |
| 9a | 109 | 61 | 1.8 |
| 9b | 105 | 73 | 1.4 |
| 9d | 93 | 70 | 1.3 |
| 9e | 102 | 102 | 1.0 |
| 9f | 106 | 106 | 1.0 |
| 11b | 102 | 102 | 1.0 |
| 11e | 99 | 99 | 1.0 |
| 11f | 103 | 103 | 1.0 |
| 12a e [11] | 90 | 27 | 3.4 |
| 12d e [11] | 42 | 4.0 | 10 |
| 12e e [11] | 27 | 10 | 2.8 |
| coumarin | >500 | >150 | - |
| imidazole | >500 | >150 | - |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsay, S.-C.; Lin, S.-Y.; Huang, W.-C.; Hsu, M.-H.; Hwang, K.C.; Lin, C.-C.; Horng, J.-C.; Chen, I.-C.; Hwu, J.R.; Shieh, F.-K.; et al. Synthesis and Structure-Activity Relationships of Imidazole-Coumarin Conjugates against Hepatitis C Virus. Molecules 2016, 21, 228. https://doi.org/10.3390/molecules21020228
Tsay S-C, Lin S-Y, Huang W-C, Hsu M-H, Hwang KC, Lin C-C, Horng J-C, Chen I-C, Hwu JR, Shieh F-K, et al. Synthesis and Structure-Activity Relationships of Imidazole-Coumarin Conjugates against Hepatitis C Virus. Molecules. 2016; 21(2):228. https://doi.org/10.3390/molecules21020228
Chicago/Turabian StyleTsay, Shwu-Chen, Shu-Yu Lin, Wen-Chieh Huang, Ming-Hua Hsu, Kuo Chu Hwang, Chun-Cheng Lin, Jia-Cherng Horng, I-Chia Chen, Jih Ru Hwu, Fa-Kuen Shieh, and et al. 2016. "Synthesis and Structure-Activity Relationships of Imidazole-Coumarin Conjugates against Hepatitis C Virus" Molecules 21, no. 2: 228. https://doi.org/10.3390/molecules21020228
APA StyleTsay, S.-C., Lin, S.-Y., Huang, W.-C., Hsu, M.-H., Hwang, K. C., Lin, C.-C., Horng, J.-C., Chen, I.-C., Hwu, J. R., Shieh, F.-K., Leyssen, P., & Neyts, J. (2016). Synthesis and Structure-Activity Relationships of Imidazole-Coumarin Conjugates against Hepatitis C Virus. Molecules, 21(2), 228. https://doi.org/10.3390/molecules21020228