Quality Evaluation of Traditional Chinese Medicine Compounds in Xiaoyan Lidan Tablets: Fingerprint and Quantitative Analysis Using UPLC-MS
Abstract
:1. Introduction
2. ExperimentalSection
2.1. Plant Materials and Reagents

2.2. Apparatus and Analytical Methods
2.3. Sample Preparation
2.4. Data Analyses
3. Results and Discussion
3.1. Optimization of Extraction and UPLC Conditions
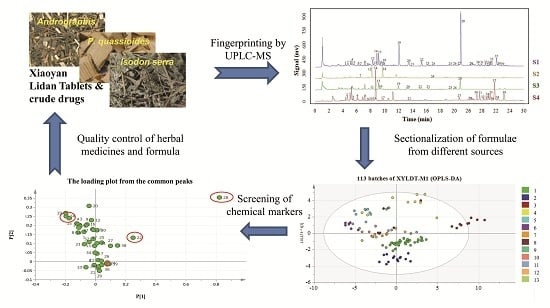
3.2. UPLC Fingerprinting of XYLDT Samples and Crude Herbs

3.3. Identification of the Structures of the Main Herb Markers Using ESI-MS
| Peak Number | RT | MS (m/z) [M + H]+ | MS2 (m/z) [M + H]+ | Compounds |
|---|---|---|---|---|
| Picrasmaquassioides | ||||
| 2 | 5.43 | 251 | 168 | 3-Methylcanthin-5,6-dione |
| 5 | 6.45 | 243 | 213 | 1-ethyl-4-Methoxy-8-hydroxycanthin-β-carboline |
| 9 | 8.66 | 257 | 197 | 4,8-Dimethoxyl-1-ethyl-carboline |
| 14 | 9.67 | 267 | 168 | 4-Methoxy-5-hydroxycanthin-6-one |
| 23 | 16.89 | 281 | 237 | 4,5-Dimethoxycanthin-6-one |
| 27 | 21.74 | 241 | 213 | 1-Ethenyl-4-methoxy-8-hydroxycanthin-β-carboline |
| 29 | 22.51 | 481 | 253 | Picradidine H |
| 30 | 24.04 | 495 | 253 | Picradidine C |
| 31 | 24.85 | 225 | 197 | 1-Ethenyl-4-methoxy-β-carboline |
| 33 | 25.81 | 479 | 267 | Picradidine F |
| 37 | 26.86 | 255 | 197 | 1-Ethenyl-4,8-dimethoxyl-β-carboline |
| Andrographis | ||||
| 15 | 10.16 | 351 | 211 | Andrographolide |
| 22 | 17.55 | 481 | 237 | Neoandrographolide |
| 28 | 22.13 | 333 | 297 | Dehydroandrographolide |
| Isodonserra | ||||
| 24 | 17.57 | 361 | 139 | Rosmarinic acid |
| others | - | - | - | Unknown |
3.4. SA and PCA


3.5. Analytical Characterizationof the Quantitative Analysis
| Compound | Precision (n = 6) | Reproducibility (n = 6) | Stability (n = 6) | |||
|---|---|---|---|---|---|---|
| Content (mg·Tab−1) | RSD (%) | Content (mg·Tab−1) | RSD (%) | Content (mg·Tab−1) | RSD (%) | |
| 4-Methoxy-5-hydroxycanthin-6-one | 0.5273 | 2.94 | 0.5394 | 2.78 | 0.5414 | 2.61 |
| Andrographolide | 8.4279 | 0.11 | 8.3933 | 0.54 | 8.4387 | 0.18 |
| Dehydroandrographolide | 4.4402 | 0.20 | 4.5892 | 1.55 | 4.5711 | 0.80 |
| Neoandrographolide | 2.5032 | 2.14 | 2.5326 | 1.68 | 2.4955 | 1.70 |
| Rosmarinic acid | 2.2165 | 0.30 | 2.2200 | 0.66 | 2.2264 | 0.62 |
| Compound | Original (mg) | Spiked (mg) | Found (mg) | Recovery (%) | Average Recovery (%) | RSD (%) (n= 3) |
|---|---|---|---|---|---|---|
| 4-Methoxy-5-hydroxycanthin-6-one | 0.3236 | 0.1511 | 0.4827 | 103.7 | 102.4 | 2.54 |
| 0.3022 | 0.6304 | 100.0 | ||||
| 0.4533 | 0.7966 | 103.4 | ||||
| Andrographolide | 5.0360 | 2.5444 | 7.6376 | 100.8 | 100.1 | 1.32 |
| 5.0888 | 10.2118 | 100.3 | ||||
| 7.6332 | 12.6637 | 99.1 | ||||
| Dehydroandrographolide | 2.7535 | 1.3812 | 4.1851 | 102.2 | 103.3 | 1.53 |
| 2.7624 | 5.6323 | 102.8 | ||||
| 4.1436 | 7.1293 | 104.8 | ||||
| Neoandrographolide | 1.5196 | 0.7509 | 2.2602 | 97.2 | 97.2 | 1.02 |
| 1.5018 | 3.0400 | 99.8 | ||||
| 2.2527 | 3.8956 | 104.6 | ||||
| Rosmarinic acid | 1.3320 | 0.6505 | 1.9989 | 101.0 | 100.9 | 2.44 |
| 1.3010 | 2.6874 | 98.0 | ||||
| 1.9515 | 3.2809 | 102.1 |
| Compound | Regression Equation (Y = ax + b) | r2 | Linear Range (μg·mL−1) |
|---|---|---|---|
| 4-Methoxy-5-hydroxycanthin-6-one | Y = 97672x − 5.023 | 0.9999 | 0.50–32.16 |
| Andrographolide | Y = 31564x − 6.875 | 0.9999 | 10.00–160.02 |
| Dehydroandrographolide | Y = 37545x − 10.8 | 1.0000 | 40.13–200.65 |
| Neoandrographolide | Y = 27057x + 19.806 | 1.0000 | 17.03–122.64 |
| Rosmarinic acid | Y = 26300x + 1.9303 | 0.9999 | 1.91–61.25 |
3.6. Quantitation of 4-Methoxy-5-hydroxycanthin-6-one, RosmarinicAcid, Andrographolide, Dehydroandrographolide and Neoandrographolide

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations:
| XYLDTs | XiaoyanLidan tablets |
| UPLC | ultra-performance liquid chromatography |
| PDA | photodiode array |
| SA | similarity analysis |
| PCA | principal component analysis |
References
- China Medical Science Press. Pharmacopoeia of People’s Republic of China; China Medical Science Press: Beijing, China, 2010; Volume 1, pp. 1031–1032. [Google Scholar]
- Abu-Ghefreh, A.; Canatan, H.; Ezeamuzie, C. In vitro and in vivo anti-inflammatory effects of andrographolide. Int. Immunopharmacol. 2009, 9, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Suebsasana, S.; Pongnaratorn, P.; Sattayasai, J.; Arkaravichien, T.; Tiamkao, S.; Aromdee, C. Anti-inflammatory and toxic effects of andrographolide derivatives in experimental animals. Arch. Pharmacol. Res. 2009, 32, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; He, E.; Wang, L.; Liu, K. Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF level. Asian Nat. Prod. Res. 2008, 10, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, C.; Chou, W. Andrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effect. Br. J. Pharmacol. 2002, 135, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, P.; Zhang, G.; Xu, L.; Wang, D.; Wang, L. Design, synthesis and antibacterial activity of novel andrographolide derivatives. Bioorg. Med. Chem. 2010, 18, 4269–4274. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, P.; Jiang, J.; Zhang, Z.; Wang, Z. Synthesis and evaluation of antibacterial activities of andrographolide analogues. Eur. J. Med. Chem. 2009, 44, 2936–2943. [Google Scholar] [CrossRef] [PubMed]
- Kapil, A.; Koul, I.B.; Banerjee, S.K.; Gupta, B.D. Antihepatotoxic effects of major diterpenoid constituents of Andrographis paniculata. Biochem. Pharmacol. 1993, 46, 182–185. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, Z.; Zhang, R.; Zhang, X.; Lu, Y.; Yang, J. Protective effect of andrographolide against concanavalin A-induced liver injury. Arch. Pharmacol. 2012, 385, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, S.; Nyavanandi, V.; Thunuguntla, S.; Kasu, S.; Pallerla, M.; Ram, P. Synthesis and structure–activity relationships of andrographolide analogues as novel cytotoxic agents. Bioorg. Med. Chem. Lett. 2004, 14, 4711–4717. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Yun, B.; Xiao, Y.; Chang, A. Synthesis, structure–activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorg. Med. Chem. Lett. 2014, 10, 2353–2359. [Google Scholar]
- Kamdem, R.; Sang, S.; Ho, C. Mechanism of the superoxide scavenging activity of neoandrographolide-A aatural product from Andrographis paniculata Nees. Agric. J. Food Chem. 2002, 50, 4662–4665. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhao, F.; Liu, Z.; Li, J.; Qiu, F. Microbial transformation of neoandrographolide by Mucorspinosus. J. Mol. Catal. B Enzym. 2011, 1, 83–88. [Google Scholar] [CrossRef]
- Yang, J.; Song, Z. (Eds.) Picrasma quassioides(D. Don) Benn. In Modern Research of Traditional Chinese Herbs; Beijing Medical University & Beijing Union Medical College Press: Beijing, China, 1996; pp. 498–522.
- Sung, Y.; Koike, K.; Nikaido, T.; Ohmoto, T.; Sankawa, U. Inhibitors of cyclic AMP phosphodiesterase in Picrasma quassioides Bennet, and inhibitory activities of related beta-carboline alkaloids. Chem. Pharm. Bull. 1984, 5, 1872–1877. [Google Scholar] [CrossRef]
- Ohmoto, T.; Sung, Y.; Koike, K. Effect of alkaloids of simaroubaceous plants on the local blood flow rate. Shoyakugaku Zasshi. 1985, 39, 28–34. [Google Scholar]
- Liu, J.; Shao, M.; Zhai, D.; Liu, K.; Wu, L. Protective effect of 4-methoxy-5-hydroxycanthin-6-one, a natural alkaloid, on dextran sulfate sodium-induced rat colitis. Planta Med. 2009, 2, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Qi, D.; Yang, M.; Fang, H.; Liu, K.; Zhao, F. In vitro and in vivo anti-inflammatory effects of 4-methoxy-5-hydroxycanthin-6-one, a natural alkaloid from Picrasma quassioides. Phytomedicine 2013, 20, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Shinji, K.; Abdelfatteh, O.; Junkyu, H.; Hiroko, I. Antidepressant-like effects of rosmarinic acid through mitogen-activated protein kinase phosphatase-1 and brain-derived neurotrophic factor modulation. J. Funt. Foods 2015, 14, 758–766. [Google Scholar]
- Sun, H.; Deng, W.; Zhan, L.; Xu, L. Effect of Banlangen granule on mice challenged withA/California/7/2009. Chin. J. Compar. Med. 2010, 20, 53–56. [Google Scholar]
- Cheng, X.; Zhao, T.; Yang, T.; Wang, C.; Annie, S.; Wang, Z. HPLC fingerprints combined principal component analysis, hierarchical cluster analysis and linear discriminant analysis for the classification and differentiation of Pegaum sp. indigenous to China. Phytochem. Anal. 2010, 21, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, Y.; Lei, P.; Cai, Z.; Liu, H. Chromatographic fingerprint study on Evodia rutaccarpa (Juss.) Benth by HPLC-DAD-ESI-MSn. J. Sep. Sci. 2010, 33, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Bai, Y.; Zhang, Q.; Zhao, Y. Chromatographic fingerprint analysis of Semen Ziziphi Spinosae by HPLC-DAD method. Anal. Lett. 2009, 42, 205–215. [Google Scholar] [CrossRef]
- Jelena, A.; Gjoshe, S.; Ivana, C.; Rumenka, P. Fingerprinting of morphine using chromatographic purity profiling and multivariate data analysis. J. Pharm. Biomed. Anal. 2015, 109, 18–27. [Google Scholar]
- Davood, M.; Milad, I.; Abolfazl, S.; Azar, H. Cytotoxicity evaluation of extracts and fractions of five marinesponges from the Persian Gulf and HPLC fingerprint analysis of cytotoxic extracts. Asian Pac. J. Trop. Biomed. 2015, 5, 896–901. [Google Scholar]
- Fraige, M.; Pereira, E.R.; Carrilho, E. Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC–DAD–MS and exploratory analysis by principal component analysis. Food Chem. 2014, 145, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Pan, M.; Dai, Y.; Chen, Y. Study on HPLC fingerprint of three TCM prescription named Xiaoyanlidan Tablet in Market. Chin. Pharm. J. 2010, 45, 1530–1534. [Google Scholar]
- Jin, Y.; Sun, Y.; Wang, X.; Xu, H. Simultaneously quantitative determination of six chemical compositions in XiaoyanLidan Pian by HPLC-MS. J. Beijing Univ. Tradit. Chin. Med. 2013, 2, 124–128. [Google Scholar]
- Shi, Y.; Xie, Z.; Wang, R.; Huang, S. Chromatographic fingerprint study on water-soluble extracts of Radix isatidis, foliumisatidis, and their preparations by HPLC-DAD technique. J. Liq. Chromatogr. R. T. 2013, 36, 80–93. [Google Scholar]
- Lai, Z. Studies on Chemical Constituents and Quality of Picrasma quassioides. Master Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2011. [Google Scholar]
- Cao, S.; Zu, Y.; Zhang, L. Simultaneous determination of four major volatile components in rosemary (Rosmarnus officinalis L.) leaves by LC-MS/MS with ultrasonic-assisted extraction. Food Sci. 2012, 33, 196–200. [Google Scholar]
- Deng, W.; Nie, R.; Liu, J. Pharmacological effects comparison of four kinds of andrographolide. Exp. Res. 1982, 17, 195–198. [Google Scholar]
- Sample Availability: Samples of the compounds andrographolide, dehydroandrographolide, neoandrographolide, rosmarinic acid and 4-methoxy-5-hydroxycanthin-6-one are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Xiong, A.; Wang, R.; Yang, L.; Wang, Z. Quality Evaluation of Traditional Chinese Medicine Compounds in Xiaoyan Lidan Tablets: Fingerprint and Quantitative Analysis Using UPLC-MS. Molecules 2016, 21, 83. https://doi.org/10.3390/molecules21020083
Yang N, Xiong A, Wang R, Yang L, Wang Z. Quality Evaluation of Traditional Chinese Medicine Compounds in Xiaoyan Lidan Tablets: Fingerprint and Quantitative Analysis Using UPLC-MS. Molecules. 2016; 21(2):83. https://doi.org/10.3390/molecules21020083
Chicago/Turabian StyleYang, Na, Aizhen Xiong, Rui Wang, Li Yang, and Zhengtao Wang. 2016. "Quality Evaluation of Traditional Chinese Medicine Compounds in Xiaoyan Lidan Tablets: Fingerprint and Quantitative Analysis Using UPLC-MS" Molecules 21, no. 2: 83. https://doi.org/10.3390/molecules21020083
APA StyleYang, N., Xiong, A., Wang, R., Yang, L., & Wang, Z. (2016). Quality Evaluation of Traditional Chinese Medicine Compounds in Xiaoyan Lidan Tablets: Fingerprint and Quantitative Analysis Using UPLC-MS. Molecules, 21(2), 83. https://doi.org/10.3390/molecules21020083